

Carlos Alexandre de Amorim Garcia1; Álisson Giovani Freitas de Oliveira2; Carlos Eduardo Nunes de Lima3; Fabiano Nunes Rocha4; Carlos Alexandre de Amorim Garcia Filho5
DOI: 10.1590/S0004-27492006000500002
ABSTRACT
PURPOSE: To describe retinal nerve fiber layer changes in late-stage diffuse unilateral subacute neuroretinitis eyes and compare these results with healthy eyes observed through nerve fiber analyzer (GDx®). METHODS: This is a retrospective case-control study in which 49 eyes in late-stage diffuse unilateral subacute neuroretinitis were examined from May/97 to December/01. First, eyes with diffuse unilateral subacute neuroretinitis and healthy contralateral eyes (Control Group I) were statistically matched. Subsequently, eyes with diffuse unilateral subacute neuroretinitis were compared with eyes of healthy patients (Control Group II). RESULTS: Eyes from Control Groups I and II had higher relative frequency of "within normal limits" status. Eyes from the diffuse unilateral subacute neuroretinitis (DUSN) Group had higher frequency of "outside normal limits" and "borderline" status. Control Groups I and II had absolute values different from the DUSN Group regarding all parameters (p<0.05), except for Symmetry in Control Groups I and II, Average thickness and Superior Integral in control group II. CONCLUSION: Patients with late-stage diffuse unilateral subacute neuroretinitis presented presumed decrease in nerve fiber layer thickness shown by GDx®. Retinal zones with larger vascular support and larger amount of nerve fibers presented higher decrease in the delay of the reflected light measured by the nerve fiber analyzer.
Keywords: Electroretinography; Evoked potentials, visual; Optic neuritis; Retinitis; Optic nerve diseases; Nerve fibers; Lasers; Perimetry; Uveitis; posterior
RESUMO
OBJETIVO: Descrever as alterações observadas na camada de fibras nervosas da retina de olhos portadores de neurorretinite subaguda unilateral difusa (DUSN) em fase crônica e compará-las aos valores obtidos em olhos normais pelo analisador de fibras nervosas da retina (GDx®). MÉTODOS: Trata-se de estudo retrospectivo caso-controle, no qual foram avaliadas a camada de fibras nervosas retiniana (CFNR) de 49 olhos portadores da doença em fase crônica examinados entre maio/97 e dezembro/01. Os olhos com neurorretinite subaguda unilateral difusa foram comparados estatisticamente com olhos normais contralaterais (Grupo Controle I) e com olhos de pacientes sem doença ocular (Grupo Controle II). RESULTADOS: Os olhos dos grupos Controle I e II tiveram maior freqüência do status "within normal limits". Os olhos com neurorretinite subaguda unilateral difusa tiveram maior freqüência relativa dos status "outside normal limits" e "borderline". Os grupos Controle I e II apresentaram valores absolutos diferentes do Grupo Doente em praticamente todos os parâmetros testados (p<0,05), as exceções foram o parâmetro "Symetry" em relação aos 2 grupos controles, e dos parâmetros "Average thickness" e "Superior integral" com relação ao Grupo Controle II. CONCLUSÃO: Os pacientes acometidos por neurorretinite subaguda unilateral difusa em fase crônica apresentaram diminuição da espessura da neurorretinite subaguda unilateral difusa demonstrada ao GDx®. As áreas da retina que contam com maior suporte vascular e maior quantidade de fibras nervosas apresentaram maior diminuição no retardo da luz medido pelo analisador de fibras nervosas.
Descritores: Eletrorretinografia; Potenciais evocados visuais; Neurite óptica; Retinite; Doenças do nervo óptico; Fibras nervosas; Lasers; Perimetria; Uveíte posterior
ORIGINAL ARTICLE
Retinal nerve fiber layer analysis using GDx® in 49 patients with chronic phase DUSN
Análise da camada de fibras nervosas em 49 casos de DUSN na fase tardia usando GDx®
Carlos Alexandre de Amorim GarciaI; Álisson Giovani Freitas de OliveiraII; Carlos Eduardo Nunes de LimaIII; Fabiano Nunes RochaIV; Carlos Alexandre de Amorim Garcia FilhoV
IProfessor, PhD, in Opthalmology, Universidade Federal do Rio Grande do Norte - UFRN - Natal (RS) - Brasil
IIMedical Resident in Opthalmology, UFRN - Natal (RS) - Brasil
IIIMedical Resident in Opthalmology, UFRN - Natal (RS) - Brasil
IVFellow, Retina Sector da UFRN - Natal (RS) - Brasil
VUndergraduate, Course of Medicine, UFRN - Natal (RS) - Brasil
ABSTRACT
PURPOSE: To describe retinal nerve fiber layer changes in late-stage diffuse unilateral subacute neuroretinitis eyes and compare these results with healthy eyes observed through nerve fiber analyzer (GDx®).
METHODS: This is a retrospective case-control study in which 49 eyes in late-stage diffuse unilateral subacute neuroretinitis were examined from May/97 to December/01. First, eyes with diffuse unilateral subacute neuroretinitis and healthy contralateral eyes (Control Group I) were statistically matched. Subsequently, eyes with diffuse unilateral subacute neuroretinitis were compared with eyes of healthy patients (Control Group II).
RESULTS: Eyes from Control Groups I and II had higher relative frequency of "within normal limits" status. Eyes from the diffuse unilateral subacute neuroretinitis (DUSN) Group had higher frequency of "outside normal limits" and "borderline" status. Control Groups I and II had absolute values different from the DUSN Group regarding all parameters (p<0.05), except for Symmetry in Control Groups I and II, Average thickness and Superior Integral in control group II.
CONCLUSION: Patients with late-stage diffuse unilateral subacute neuroretinitis presented presumed decrease in nerve fiber layer thickness shown by GDx®. Retinal zones with larger vascular support and larger amount of nerve fibers presented higher decrease in the delay of the reflected light measured by the nerve fiber analyzer.
Keywords: Electroretinography; Evoked potentials, visual; Optic neuritis; Retinitis; Optic nerve diseases; Nerve fibers; Lasers/diagnostic use; Perimetry; Uveitis, posterior
RESUMO
OBJETIVO: Descrever as alterações observadas na camada de fibras nervosas da retina de olhos portadores de neurorretinite subaguda unilateral difusa (DUSN) em fase crônica e compará-las aos valores obtidos em olhos normais pelo analisador de fibras nervosas da retina (GDx®).
MÉTODOS: Trata-se de estudo retrospectivo caso-controle, no qual foram avaliadas a camada de fibras nervosas retiniana (CFNR) de 49 olhos portadores da doença em fase crônica examinados entre maio/97 e dezembro/01. Os olhos com neurorretinite subaguda unilateral difusa foram comparados estatisticamente com olhos normais contralaterais (Grupo Controle I) e com olhos de pacientes sem doença ocular (Grupo Controle II).
RESULTADOS: Os olhos dos grupos Controle I e II tiveram maior freqüência do status "within normal limits". Os olhos com neurorretinite subaguda unilateral difusa tiveram maior freqüência relativa dos status "outside normal limits" e "borderline". Os grupos Controle I e II apresentaram valores absolutos diferentes do Grupo Doente em praticamente todos os parâmetros testados (p<0,05), as exceções foram o parâmetro "Symetry" em relação aos 2 grupos controles, e dos parâmetros "Average thickness" e "Superior integral" com relação ao Grupo Controle II.
CONCLUSÃO: Os pacientes acometidos por neurorretinite subaguda unilateral difusa em fase crônica apresentaram diminuição da espessura da neurorretinite subaguda unilateral difusa demonstrada ao GDx®. As áreas da retina que contam com maior suporte vascular e maior quantidade de fibras nervosas apresentaram maior diminuição no retardo da luz medido pelo analisador de fibras nervosas.
Descritores: Eletrorretinografia; Potenciais evocados visuais; Neurite óptica; Retinite; Doenças do nervo óptico; Fibras nervosas; Lasers/uso diagnóstico; Perimetria; Uveíte posterior
INTRODUCTION
Diffuse unilateral subacute neuroretinitis (DUSN) is an ocular disease caused by a nematode(1). It affects the retina, especially the posterior pole, and is one of the main causes of unilateral blindness in Northeast Brazil(2). The clinical course is characterized by periods of activity and remission. The acute phase consists of focal retinitis and vitreitis associated with inflammatory edema of the optic disk. In the chronic phase, if the nematoid is not destroyed, atrophy of the optic nerve, diffuse retinal degeneration and vascular narrowing occur(3-4). Early diagnosis and treatment are fundamental for preserving vision in the affected eyes(1).
The GDx nerve fiber analyzer (Laser Diagnostic Technologies, Inc) is a scanning confocal laser polarimeter, which uses a polarized light source to analyze the retinal nerve fiber layer (RNFL) thickness by measuring the delay of emitted light(5). The device was originally created to analyze retinal nerve fiber layer changes secondary to glaucomatous optic neuropathy(6-8). Some studies have been performed in order to establish a correlation between GDx® and nerve fiber layer changes resulting from non-glaucomatous processes(5,9-16). Miyahara et al., verified a momentary increase in nerve fiber layer thickness, followed by a definitive, progressive decrease in patients with traumatic optic neuropathy(15). Lester et al., used the nerve fiber analyzer in an attempt to verify RNFL changes during the momentary increase (45 seconds) of intraocular pressure resulting from suction in the Lasik process(5). Tatlipinar et al. showed that druses visible at the head of the optic nerve lead to a decrease in RNFL delay(16).
The purpose of this study is to demonstrate the RNFL changes observed through polarimetry in chronic-phase DUSN eyes.
METHODS
This is a retrospective case-control study, in which the examinations of retinal nerve fiber layer of 49 patients with clinical diagnosis of chronic-phase DUSN and 16 normal patients were analyzed and evaluated from May/97 to December/01 and in February/04, respectively.
All patients were submitted to applanation tonometry, gonioscopy and optic nerve evaluation through fundus biomicroscopy. None of these presented changes suggestive or suspected of glaucoma.
All DUSN patients were classified in the chronic phase according to the following criteria: optic nerve atrophy, diffuse retinal epithelium pigmentary atrophy, mild or moderate vitreitis, afferent pupillary defect, multifocal choroiditis episodes, increase in the internal limiting membrane reflex (Oréfice's sign), the presence of small white spots suggestive of calcification and evidence of tunnels in the subretinal space (Garcia's signs)(3).
All patients were submitted to the following technique to capture images in the Nerve Fiber Analyzer System, GDx®, Laser Diagnostic Technologies, Inc LDT P/N 591-0029B: ambient lights on, absence of medicamentous mydriasis, 3-image capture for each eye during the examination, exclusion of examinations which presented movement artifacts or off-centered optic disk. Images were considered valid if they presented an overall score greater than 90 (automatic evaluation of the device- software version 2.0). Patients under 18 years had their ages adjusted for this value (the minimum accepted in the device's data bank).
From the examinations performed between May/97 and December/01 the case (49 DUSN eyes) and control I (49 contralateral eyes) groups were determined. In February/04 control group II, consisting of 16 patients (32 eyes) without ocular disease, was evaluated.
Status denominators (within normal limits, borderline and outside normal limits) were obtained and analyzed, calculating the frequency of the data obtained for each GDx® parameter in DUSN eyes. A comparison was established between the qualitative and quantitative frequencies in the three groups (diseased, control I and control II). The quantitative frequencies were provided by the following parameters: symmetry, superior ratio, inferior ratio, superior/nasal, max. modulation, the number, average thickness, ellipse average, superior average and superior integral. Some patients underwent more than one examination at different time intervals, but only the first examination with reproductibility and software approval was considered.
Paired t test was used to evaluate statistical significance when comparing control group I and the case group. When comparing control group II and the case group a difference was observed between the number of eyes in each group. In this case, t test for independent samples was used.
For a better understanding of the examination, all analyzed parameters are briefly explained.
Superior/nasal ratio is the result of the ratio between average thickness of the 1,500 most delayed points in the superior quadrant and of the 1,500 points nearest to the median value of the nasal sector.
Maximum modulation is obtained through an equation which involves: (1) the average of 1,500 points peripheral to the ellipse with the greatest delay in the superior and inferior quadrants; (2) the lowest value obtained when calculating the average of the 1,500 points peripheral to the ellipse of nearest value to the median in the nasal and temporal quadrants.
Ellipse modulation is the modulation of the points inside the ellipse. It is calculated similarly to maximum modulation, but only the 200 points measured inside the ellipse are used.
Superior ratio is the ratio between the average thickness of the 1,500 pixels of greatest delay in the superior quadrant and of the 1,500 points nearest to the median value in the temporal sector.
Inferior ratio is the ratio between the average thickness of the 1,500 pixels of greatest delay in the inferior quadrant and of the 1,500 points nearest in value to the median in the temporal sector.
Average thickness is the average of all 65,536 pixels with valid measures.
Ellipse average is the average of points inside the 10-pixel band delimited by the ellipse.
Superior average is the average of points inside the ellipse, located in the superior sector.
Inferior average is the average of points inside the ellipse, located in the inferior sector.
Superior integral is the total area below the curve of the nerve fiber layer graph regarding the ellipse points in the superior sector.
The number consists of the analysis of approximately 130 variables through the neural calculation of retrograde propagation.
RESULTS
Of 49 evaluated DUSN patients, the mean age was 24.22± 10.66 years (range= 8-48 years) (mode – 18 years).
In the case group, the right eye was affected in 57.1% of the cases. Thirty-three patients (67.3%) were males.
Table 1 shows that the frequency of "within normal limits" was higher in control groups 1 and 2 when compared to the DUSN group in practically all the parameters and that the frequency of de "borderline" and "outside normal limits" was greater in the DUSN group.
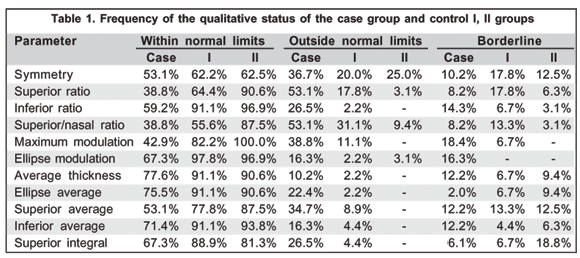
In control group II, the mean age was 33.3±10.81 years (range=19-46 years), (mode - 27 years). The frequency of the qualitative results of this group is illustrated in Table 1.
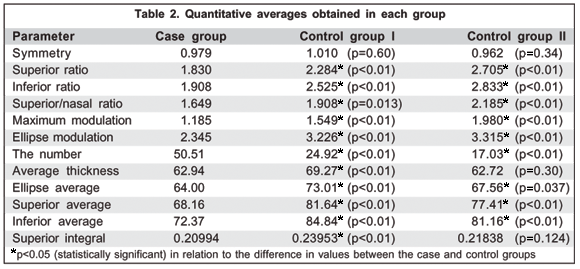
Table 2 presents averages obtained for each parameter in the 3 groups. It was observed that the mean of these values was greater in the control groups compared to the DUSN group. A statistically significant difference was found in practically all the parameters assessed (p<0.05), except for symmetry, average thickness and superior integral when comparing control group II and the DUSN group.
DISCUSSION
Only one case of strongly suspected clinical DUSN was histopathologically studied by Gass and Scelfo (1978)(17). The eye showed evidence of a non-specific inflammatory process, involving the vitreous body, optic nerve, retina and choroid. Histopathologic data were not sufficient to explain visual loss in this case, which contributed to speculation about the role of functional mechanisms in causing visual damage(3).
There are no reports suggesting a preferred region for the onset of the changes. The disease is characterized by diffuse inflammatory damage to the neuroretina. It presents a very characteristic and reproducible electroretinographic picture: negative electroretinogram (ERG) (b wave of maximum combined response is flat, with below normal response and a decrease in relation to b/a). This type of ERG is typically found in ischemic retinal cases(3). The mechanism of this interesting phenomenon is explained by Oréfice and Gonçalves as being a consequence of a possible autoimmune, inflammatory and/or toxic aggression towards retinal bipolar cells(3). These changes are found in the chronic phase and are similar to other ischemic retinal cases where information on characterizing DUSN is limited(3).
The case group consisted of young patients, which confirms literature data(1,18).
In the researched literature (Pubmed, Medline, LILACS, SciELO, Webof Science) no reports on the use of GDx® in patients with DUSN were encountered. This device is used as a diagnostic tool in suspected glaucoma patients. Its conception is based on the natural history of glaucoma, which initially manifests itself through localized RNFL losses. Available studies show that the parameters with the best sensitivity/specificity ratio for glaucoma diagnosis, according to the internal data bank of the device, are: superior/nasal ratio and the number(19). The building of receiver operating characteristic (ROC) curves with new cutoff points increased this relation and optimized the following parameters: ellipse modulation, maximum modulation, superior/nasal and the number(20). It is worth emphasizing that the majority of these parameters deal with the comparison between the vertical and horizontal RNFL regions(20).
After comparing the 3 study groups, we can divide the parameters that present statistically significant differences into:
1) Parameters based on comparative ratios between the vertical and horizontal regions
It was observed that the comparative ratios between the vertical and horizontal regions presented values directly proportional to the vertical retinal and inversely proportional to horizontal retinal damage, respectively. It was verified that, although it is a diffuse disease, DUSN causes a greater decrease in delay and RNFL thickness in areas with higher vascular supply and larger amount of nerve fibers. Thus, damaged eyes present parameters with lower values than those found in normal eyes.
Alterations in these parameters are expected during glaucoma investigation because the glaucomatous optic neuropathy is characterized by a loss pattern that initiates in the vertical region (first, inferior, then superior) and afterwards in the horizontal region (the temporal quadrant is the last to suffer loss)(21-22).
2) Parameters based on calculating average delay
The calculation of the average delay does not depend on the comparison between meridians. Thus, the decrease in its value is directly proportional to the diffuse lesion caused by DUSN, leading to lower results in the case group than in the control group.
The parameters that do not depend on the comparison between the vertical and horizontal meridians are proportional to the resulting global lesion. They are not the most sensitive indices for early glaucoma diagnosis. At disease onset, loss occurs at the vertical meridian and tends to spare the horizontal fibers (global means are scarcely affected). Nevertheless, these indices become useful in monitoring disease progression in more advanced cases (the more advanced the disease, the lower the mean delay)(20).
3) Parameters not related to averages or comparisons between regions
Eyes with DUSN presented much lower values for superior integral due to the diffuse loss that the disease causes in RNFL. The case group presented a higher average value for the number than the control groups. This difference may suggest greater RNFL loss in this group. In glaucoma, the superior integral parameter is altered when RNFL loss affects that quadrant(20). If the disease causes loss only in the inferior quadrant, for example, it will not be useful in early diagnosis. Nevertheless, it may be useful in cases in which vertical damage reaches the superior quadrant. Its reduction is directly proportional to the resulting lesion in the fibers of this particular area. Information about the pathogenesis of the disease is, for the most part, speculative(3). Toxic products released by the larva in the subretinal space would locally affect the external portion of the retina and a diffuse tissue reaction would lead to external and internal retinal lesion. Over the years vascular narrowing and progressive ganglionar cell loss would occur until optic atrophy resulted(3).
The majority of patients are not diagnosed in the acute phase(1). The optic nerve may be normal or swollen, characterizing the onset of optic neuritis(3). Should the larva not be destroyed during this phase, it is possible to have two types of RNFL alterations: (1) increase in thickness, due to transitory edema or (2) decrease in thickness secondary to nerve fiber loss that occurs with the progression of the disease. GDx® was able to demonstrate a decrease in RNFL thickness during the chronic phase.
The fact that the device's data bank is not adjusted for ages below 18 years may lead to some bias. The correction of corneal birefringence could improve the quality of the examination.
Patients with chronic phase DUSN presented with a decrease in RNFL thickness, which can be demonstrated with GDx®. The areas of the retina that have the greatest vascular support and highest amount of nerve fibers exhibited a greater decrease in light delay measured by the nerve fiber analyzer.
CONCLUSION
Future studies are necessary to validate the use of this examination in the follow-up of patients whose larva was discovered and destroyed. This is especially important for patients whose larva was not found and who only underwent clinical treatment, so that the progression of the disease may be monitored.
REFERENCES
1. Garcia CA, Gomes AH, Garcia Filho CA, Vianna RN. Early-stage diffuse unilateral subacute neuroretinitis: improvement of vision after photocoagulation of the worm. Eye. 2004;18(6):624-7.
2. Garcia CAA, Gomes AHB, Barbosa MFA, Rocha MLR, Uchôa RAC. Aspectos clínicos e epidemiológicos das uveítes em Natal - RN. Rev Bras Oftalmol. 2002;61(2):121-9.
3. Oréfice F, Bonfioli AA, Paranhos FRL. Neuroretinite subaguda unilateral difusa. In: Oréfice F. Uveíte: clínica e cirúrgica. Rio de Janeiro: Cultura Médica; 1998. p.733-56.
4. Gass JD, Braunstein RA. Further observations concerning the diffuse unilateral subacute neuroretinitis syndrome. Arch Ophthalmol. 1983;101(11):1689-97.
5. Lester M, Tizte P, Mermoud A. Retinal nerve fiber layer thickness changes after an acute increase in intraocular pressure. J Cataract Refract Surg. 2002;28 (12):2117-22. Comment in: J Cataract Refract Surg. 2003;29(12):2255-7; author reply 2257-8.
6. Weinreb RN, Dreher AW, Coleman A, Quigley H, Shaw B, Reiter K. Histopathologic validation of Fourier-ellipsometry measurements of retinal nerve fiber layer thickness. Arch Ophthalmol. 1990;108(4):557-60.
7. Morgan JE, Waldock A, Jeffery G, Cowey A. Retinal nerve fibre layer polarimetry: histological and clinical comparison. Br J Ophthalmol. 1998;82(6):684-90.
8. Weinreb RN, Shakiba S, Zangwill L. Scanning laser polarimetry to measure the nerve fiber layer of normal and glaucomatous eyes. Am J Ophthalmol. 1995;119(5):627-36.
9. Banks MC, Robe-Collignon NJ, Rizzo JF 3rd, Pasquale LR. Scanning laser polarimetry of edematous and atrophic optic nerve heads. Arch Ophthalmol. 2003;121(4):484-90.
10. Bozkurt B, Irkec M, Orhan M, Karaagaoglu E. Thickness of the retinal nerve fiber layer in patients with anisometropic and strabismic amblyopia. Strabismus. 2003;11(1):1-7.
11. Colen TP, Van Everdingen JA, Lemij HG. Axonal loss in a patient with anterior ischemic optic neuropathy as measured with scanning laser polarimetry. Am J Ophthalmol. 2000;130(6):847-50.
12. Foroozan R, Buono LM, Savino PJ, Sergott RC. Scanning laser polarimetry of the retinal nerve fiber layer in central retinal artery occlusion. Ophthalmology. 2003;110(4):715-8.
13. Giampani Júnior J, Medeiros FA, Malta RFS, Susanna Júnior R. Polarimetria de varredura a laser em pacientes portadores de fibras de mielina. Rev Bras Oftalmol. 2002;61(4):259-64.
14. Medeiros FA, Dantas NC, Ginguerra MA, Susanna Junior R. Análise da camada de fibras nervosas da retina em portadores de enxaqueca com aura. Arq Brás Oftalmol. 2001;64(5):389-93.
15. Miyahara T, Kurimoto Y, Kurokawa T, Kuroda T, Yoshimura N. Alterations in retinal nerve fiber layer thickness following indirect traumatic optic neuropathy detected by nerve fiber analyzer, GDx-N. Am J Ophthalmol. 2003;136 (2):361-4.
16. Tatlipinar S, Kadayifcilar S, Bozkurt B, Gedik S, Karaagaoglu E, Orhan M, Irkec M. Polarimetric nerve fiber analysis in patients with visible optic nerve head drusen. J Neuroophthalmol. 2001;21(4):245-9.
17. Gass JD, Scelfo R. Diffuse unilateral subacute neuroretinitis J R Soc Med. 1978;71(2):95-111.
18. Souza EC. Aspectos clínicos e terapêuticos da neurorretinite unilateral difusa subaguda . São Paulo: Universidade de São Paulo; 1997.
19. Lauande-Pimentel R, Carvalho RA, Oliveira HC, Gonçalves DC, Silva LM, Costa VP. Discrimination between normal and glaucomatous eyes with visual field and scanning laser polarimetry measurements. Br J Ophthalmol. 2001;85 (5):586-91. Erratum in: Br J Ophthalmol 2002;86(6):707. Erratum in: Br J Ophthalmol 2002;86(6):707.
20. Lauande-Pimentel R, Costa VP. Análise da camada de fibras nervosas da retina. Um guia para interpretar o exame de polarimetria. Rio de Janeiro: Cultura Médica; 2001.
21. Tuulonen A, Lehtola J, Airaksinen PJ. Nerve fiber layer defects with normal visual fields. Do normal optic disc and normal visual field indicate absence of glaucomatous abnormality? Ophthalmology. 1993;100(5):587-97; discussion 597-8.
22. Jonas JB, Hayreh SS. Ophthalmoscopic appearance of the normal optic nerve head in rhesus monkeys. Invest Ophthalmol Vis Sci 2000;41(10):2978-83.
 Address for correspondence:
Address for correspondence:
Rua Ceará Mirim, 316
Natal (RN) CEP 59020-240
E-mail: [email protected]
Recebido para publicação em 21.09.2005
Última versão recebida em 09.02.2006
Aprovação em 27.02.2006
Study performed at Prontoclínica de Olhos and Department of Opthalmology, UFRN - Natal (RS) - Brasil.
Nota Editorial: Depois de concluída a análise do artigo sob sigilo editorial e com a anuência dos Drs. Mariza Toledo de Abreu e Moysés Eduardo Zajdenweber sobre a divulgação de seu nome como revisor, agradecemos sua participação neste processo.