

Elif Kiliç Kan1; Emrah Kan2; Bilge Eraydin3; Betül Gündüz1; Ozlem Eski Yucel3
DOI: 10.5935/0004-2749.2025-0184
ABSTRACT
PURPOSE: To investigate choroidal structural and vascular changes in patients with mild autonomous cortisol secretion using enhanced depth imaging optical coherence tomography and optical coherence tomography angiography.
METHODS: This cross-sectional study included 60 eyes of 30 patients with mild autonomous cortisol secretion and 60 eyes of 30 subjects with nonfunctional adenoma (controls) between February 2023 and January 2024. Subfoveal choroidal thickness, pachychoroid spectrum disease and choroidal vascularity index were evaluated using spectral-domain optical coherence tomography. Group comparisons were performed, and correlations between subfoveal choroidal thickness and clinical features were analyzed.
RESULTS: Pachyvessels were more common in patients with mild autonomous cortisol secretion than in controls (71.4% vs. 42.9%, p=0.002). The frequency of pachychoroidal spectrum disease was significantly higher in the mild autonomous cortisol secretion Group (68.3% vs. 31.7%; p<0.001). Median subfoveal choroidal thickness was 355 μm (range, 150–535) in the mild autonomous cortisol secretion Group and 297 μm (range, 162–597) in controls (p=0.014). Choroidal vascularity index was comparable between groups (p=0.072). Subfoveal choroidal thickness correlated significantly with axial length, spherical equivalent, post-1-mg dexamethasone suppression test cortisol level, and disease duration.
CONCLUSION: Patients with mild autonomous cortisol secretion exhibited greater subfoveal choroidal thickness and a higher frequency of pachychoroidal spectrum disease compared with controls, whereas stromal and vascular structural alterations were proportionally similar between groups.
Keywords: Adrenal gland neoplasms; Central serous chorioretinopathy; Choroid; Cushing syndrome; Hydrocortisone; Optical coherence tomography
INTRODUCTION
The choroid is a highly vascular layer of the eye composed primarily of blood vessels embedded within a stroma containing connective tissue, smooth muscle cells, melanocytes, mast cells, and nerves. It plays a crucial role in ocular physiology, providing the majority of the blood supply to the outer retina(1). Choroidal abnormalities – such as vascular hyperpermeability, structural changes, and thinning – are key contributors to the development and progression of several vision-threatening diseases, including polypoidal choroidal vasculopathy (PCV), age-related macular degeneration (AMD)(2), and central serous chorioretinopathy (CSCR)(3).
In recent years, advanced imaging modalities such as enhanced depth imaging optical coherence tomography (EDI-OCT) and swept-source OCT have enabled detailed in vivo visualization of the outer choroidal boundary. These advances have led to the identification of a group of disorders collectively referred to as the pachychoroid spectrum diseases (PSD). The pachychoroid spectrum encompasses conditions characterized by diffuse or focal choroidal thickening and dilation of the outer choroidal vessels, known as pachyvessels. Disorders within this spectrum include uncomplicated pachychoroid (UCP), pachychoroid pigment epitheliopathy (PPE), CSCR, pachychoroid neovasculopathy, aneurysmal type 1 neovascularization or PCV, peripapillary pachychoroid syndrome, and focal choroidal excavation. Eyes showing pachychoroid features without retinal pigment epithelium (RPE) changes, choroidal neovascularization (CNV), or polyps are classified as having UCP, whereas those with pachychoroid features and RPE changes but no subretinal fluid, CNV, or polyps are classified as PPE(4).
Recent studies have introduced the choroidal vascularity index (CVI), a parameter derived through image binarization that distinguishes stromal and luminal areas to quantify the relative vascular component of the choroid. Agrawal et al. first described CVI as a quantitative method for choroidal assessment and proposed that it may serve as a more reliable predictor than subfoveal choroidal thickness (SFCT) in evaluating choroidal diseases(5).
Growing evidence also indicates that the endocrine system influences SFCT variation(6-8).Chronic overproduction of cortisol leads to endogenous Cushing syndrome (CS), a classic manifestation of metabolic syndrome(9). Several studies have reported increased choroidal thickness (CT) and a higher prevalence of PSD in patients with CS compared with healthy controls(10).
The European Society of Endocrinology and the European Network for the Study of Adrenal Tumors have recently introduced the term mild autonomous cortisol secretion (MACS) to describe patients with adrenal incidentalomas and adrenocorticotropic hormone (ACTH)-independent cortisol hypersecretion, but without clinical features of overt CS, such as muscle weakness, skin fragility, or striae. Patients with adrenal incidentalomas are classified as having MACS when their postdexamethasone suppression test (DST) serum cortisol level exceeds 1.8 μg/dL. Adrenal incidentalomas are adrenal masses that are typically discovered incidentally during imaging performed for unrelated medical conditions(11).
We hypothesized that patients with MACS may exhibit choroidal thickening, potentially predisposing them to PSD, given the previously reported association between elevated cortisol levels and increased SFCT. Therefore, this study aimed to evaluate SFCT, CVI, and the presence of PSD in patients with MACS using spectral-domain OCT (SD-OCT) with enhanced depth imaging (EDI) mode.
Additionally, we examined correlations between SFCT and post-DST cortisol levels, adrenal adenoma size, and disease duration. To our knowledge, this is the first study to investigate PSD risk in patients with MACS using SD-OCT-derived metrics, including CVI and SFCT.
METHODS
Study participants
This cross-sectional study was conducted at the Ophthalmology and Endocrinology Clinics of the Faculty of Medicine, Ondokuz Mayıs University (OMU), between February 2023 and January 2024. The study was approved by OMU Ethics Committee and adhered to the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants. Thirty patients with MACS (aged >40 years, with adrenal adenoma and post-DST cortisol levels >1.8 μg/dL) and 30 age- and sex-matched controls (aged >40 years, with adrenal adenoma and post-DST cortisol levels <1.8 μg/dL) were included. Disease duration was defined as the interval between the initial diagnosis and study enrollment. Exclusion criteria were (1) refractive error ≥6 diopters; (2) history of glaucoma, uveitis, or retinal diseases such as diabetic retinopathy, retinal vein occlusion, AMD, or macular pucker; (3) history of ocular surgery, including intravitreal injections, phacoemulsification, or vitrectomy; and (4) media opacity compromising OCT image quality. A subgroup analysis was also performed to examine the effect of post-DST cortisol levels on SFCT. Participants (patients and controls combined) were categorized according to post–1-mg DST cortisol levels as follows: <1.8 μg/dL, 1.8–5 μg/dL, and >5 μg/dL.
Ophthalmic examination, image acquisition, and analysis
All participants underwent a comprehensive ophthalmic examination, including best-corrected visual acuity assessment, Goldmann applanation tonometry, and slit-lamp biomicroscopy of both the anterior and posterior segments. To minimize the effect of diurnal variation on SFCT, all OCT scans were obtained in the afternoon. Macular imaging was performed using the Heidelberg Spectralis OCT system (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) in G-Fast mode (25 B-scans, 768 A-scans per line, 30ºx20º field, scan depth 240 μm). Central retinal thickness (CRT) was automatically measured from the macular OCT scans (Figure 1A). For SFCT measurements, images were acquired in EDI mode (30º, 768 A-scans). Central SFCT was defined as the perpendicular distance from the hyperreflective outer border of the RPE to the choroid–scleral junction beneath the foveal center and was manually measured using the device's caliper tool (Figure 1B). On EDI-OCT images, vessels with large hyporeflective lumens in the Haller layer, accompanied by attenuation or loss of the overlying Sattler layer and choriocapillaris, were identified as pachyvessels. The definitions and classification of PSD followed previously published criteria(4). The presence of pachyvessels and the classification of PSD were independently assessed in a double-blind manner by two physicians (O.E.Y and E.K), and only concordant findings were included in the analysis. For CVI evaluation, EDI-OCT images were converted to binary format using ImageJ software (version 1.52a; Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). Choroidal vascular parameters were analyzed as described by Agrawal et al.(5). The total choroidal area (TCA) and luminal area (LA) were calculated from the binarized images, with the LA representing dark pixels corresponding to vascular lumens (Figure 2B). The stromal area (SA) was obtained by subtracting the LA from the TCA, and the CVI was determined as the ratio of LA to TCA. EDI-OCT scans were also reviewed to identify the presence and type of PSD. All SFCT measurements and CVI calculations were performed and recorded by an experienced ophthalmologist (B.E) specializing in retinal imaging.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA). A convenience sampling method was used. Sample size estimation was conducted through power analysis using MINITAB 16 statistical software. Based on the findings of a previous comparable study(12), assuming a significance level of α=0.05 and a statistical power of 90%, a minimum of 21 participants per group was required. The normality of data distribution was evaluated using the Shapiro–Wilk test. Continuous variables were compared using the Mann–Whitney U test, and categorical variables were compared using the chi-squared test. Results are presented as median (minimum–maximum), mean ± standard deviation (SD), or frequency (%), as appropriate. Spearman correlation analysis was performed to identify factors associated with SFCT. A p-value of <0.05 was considered statistically significant.
RESULTS
Demographic and clinical characteristics
A total of 60 eyes from 30 patients with MACS and 60 eyes from 30 subjects with nonfunctional adenoma (NFA) were included in the study. The mean ages of the MACS and control groups were 61.8 ± 8.2 and 60.06 ± 8.01 years, respectively (p=0.235). The male-to-female ratio was comparable between groups (p=0.239). Median disease duration was 18.5 (1–163) months in the MACS group and 15.5 (1–142) months in the control group (p=0.148). No significant differences were observed between groups in ocular parameters, including spherical equivalent, cylindrical refraction, intraocular pressure, and axial length (Table 1). The mean adrenal nodule size was significantly larger in the MACS group than in the control group (26.3 mm vs. 20.1 mm; p<0.001). Hypertension was present in 20 patients in the MACS group and 12 in the control group, with the difference reaching statistical significance (p=0.003). Table 1 summarizes the demographic and clinical characteristics of the study population.
OCT imaging
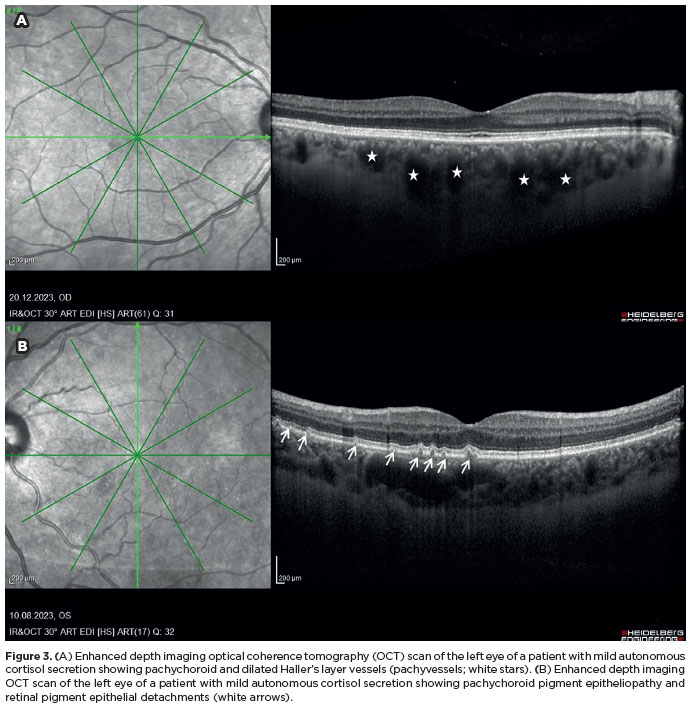
The frequency of PSD was significantly higher in the MACS group than in the control group (68.3% vs. 31.7%; p<0.001) (Figure 3B). Pachyvessels were also more common among patients with MACS than among controls (71.4% vs. 42.9%, p=0.002) (Figure 3A). The median SFCT was 355 μm (range, 150–535) in the MACS group and 297 μm (range, 162–597) in the control group, with the difference reaching statistical significance (p=0.014). No significant differences were observed between groups in CVI, TCA, LA, or SA. Comparisons of pachychoroid disorders, CVI, and related parameters between the two groups are presented in Table 2. In the subgroup analysis, higher post-1 mg DST cortisol concentrations were significantly correlated with increased SFCT (p=0.045) (Table 3).
Correlation analysis
Correlation analysis was performed to identify factors associated with CT. SFCT showed a weak negative correlation with axial length (r=−0.324, p<0.001) and weak positive correlation with spherical equivalent (r=0.255, p=0.005), post-1 mg DST cortisol level (r=0.269, p=0.003), and disease duration (r=0.309, p=0.001) (Table 4).
DISCUSSION
In the present study, we evaluated the presence of PSD, SFCT, and CVI in patients with MACS. To the best of our knowledge, this is the first study to investigate CT, CVI, and PSD in this patient group. Our findings demonstrated that SFCT and the frequency of pachychoroid disorders were significantly higher in patients with MACS than in the control group. However, no significant differences were observed in CVI, TCA, LA, or SA between the two groups.
From a clinical standpoint, it is important to recognize that MACS and overt CS represent points along a continuum of hypercortisolism. Differentiating patients with adrenal incidentaloma-related MACS from those with overt adrenal CS remains challenging both clinically and biochemically. In a recent study, Zhang et al. compared clinical features between patients with MACS and those with CS and reported that a considerable proportion of individuals with MACS exhibited proximal muscle weakness (47.5% vs. 75.0%), supraclavicular and/or dorsocervical fat accumulation (25.4% vs. 75.0%), typical skin changes (28.8% vs. 83.3%), and central obesity (39.0% vs. 83.3%)(13). Patients with MACS also have an increased risk of osteoporosis (46%) and mainly asymptomatic vertebral fractures (82%), compared with 13% and 23%, respectively, in individuals with nonfunctioning adrenal incidentalomas(14-15). However, ocular comorbidities have not been systematically investigated in patients with MACS. To date, we found only a single case report describing the clinical course of CSCR secondary to MACS before and after adrenalectomy. In that report, a 50-year-old woman presented with blurring and spots in her right eye that had persisted for several months. Bilateral, multifocal subretinal fluid and mottled pigmentary changes were detected, leading to a diagnosis of multifocal, chronic CSCR. During follow-up, the patient was found to have an adrenal incidentaloma and MACS, and the subretinal fluid resolved completely 3 months after minimally invasive adrenalectomy(16). Based on these observations, we aimed to determine whether patients with MACS develop ocular manifestations and to discuss how such findings might influence the decision between medical and surgical management. In the present study, patients with MACS exhibited a thicker choroid (355 μm vs. 297 μm) and a higher frequency of pachychoroidopathy (68.3% vs. 31.7%) compared with those with nonfunctioning adrenal incidentaloma. Demirel et al. previously hypothesized that UCP and PPE may represent prodromal stages of CSCR. Therefore, the higher prevalence of UCP and PPE observed in our patients might indicate a predisposition to subsequent development of PSD(17). Moreover, previous studies have shown that elevated cortisol concentrations after the 1-mg DST are associated with increased cardiometabolic risk and mortality(18). To assess whether cortisol excess similarly affects the choroid, we conducted a subgroup analysis and found a significant association between post-DST serum cortisol levels and SFCT (p=0.045), as well as a positive correlation between these parameters (r= 0.269, p= 0.003).
The effect of corticosteroids on CT remains controversial. Han et al. reported no significant change in CT at 1 day, 1 week, or 1 month following high-dose corticosteroid therapy, although one patient (5.6%) developed CSCR during the study period(19). Wang et al. found that SFCT was significantly correlated with 24-hour urine-free cortisol levels but not with plasma-free cortisol(10), whereas Eymard et al. observed no association between CT and urinary cortisol levels(20). These discrepancies may reflect the dynamic nature of the choroid, where fluctuations in cortisol levels might require a longer duration to induce measurable changes in CT.
Similarly, the relationship between CS and CT remains inconsistent across studies. Abalem et al. reported that patients with CS had significantly greater SFCT than healthy controls (372.96 μm vs. 255.63 μm), with one patient (9.09%) developing CSCR and another (9.09%) showing PPE(21). Karaca et al. also observed a higher SFCT in patients with CS compared to controls (367.8 μm vs. 329.0 μm) and detected bilateral, multifocal, extrafoveal CSCR in one patient (3%)(22). Consistent with these findings, Wang et al. reported significantly greater SFCT (371.6 μm vs. 320.0 μm) and a higher prevalence of PSD (53.1% vs. 14.3%) in patients with CS than in controls(10). A systematic review and meta-analysis reported that patients with CS had a 49.5-μm thicker SFCT than matched healthy individuals, with 20.8% exhibiting PPE, 7.7% exhibiting CSCR, and 2.8% exhibiting PCV(23). Similarly, Eymard et al. found no significant difference in SFCT between patients with CS and healthy controls but observed a higher presence of PSD among those with CS (21.4% vs. 3.6%), including PPE in 17.9% of eyes and PCV in 3.6%(20). Bouzas et al. reported that 5% of 60 patients with endogenous CS experienced one or more episodes of CSCR, all occurring during periods of untreated hypercortisolism when plasma cortisol concentrations were elevated(24). The present study aligns with previous findings demonstrating an association between elevated cortisol levels and increased SFCT. Patients with MACS exhibited a 58-μm thicker SFCT than the control group, supporting the hypothesis that subclinical hypercortisolism may contribute to choroidal thickening and pachychoroidopathy.
In the Beijing Eye Study, univariate regression analysis demonstrated associations between SFCT and several ocular and systemic parameters. Multivariate analysis showed that CT increased with younger age, male sex, shorter axial length, deeper anterior chamber, and thicker lens and decreased with myopic refractive error greater than −1 D(25). Karahan et al. found that CT was not significantly correlated with serum cortisol level, age, or spherical equivalent but was negatively correlated with axial length in 66 healthy volunteers without ocular disease(26). In the present study, SFCT was significantly and positively correlated with post-1 mg DST cortisol level, spherical equivalent, and disease duration and negatively correlated with axial length.
As expected, hypertension was more prevalent among patients with MACS, likely reflecting the systemic effects of elevated cortisol levels. However, the relationship between hypertension and SFCT remains controversial in the literature. Several studies have reported that hypertension is associated with reduced SFCT. Waghamare et al. found that mean SFCT was significantly lower in hypertensive individuals than in normotensive controls (253.24 ± 63.96 μm vs. 301.25 ± 55.79 μm) and negatively correlated with systolic blood pressure(27). Similarly, a meta-analysis by Papathanasiou et al. confirmed that SFCT was significantly decreased in hypertensive individuals compared with normotensive individuals(28). Conversely, other studies have found no significant association. For instance, Shao et al. reported that hypertension as a systemic condition did not significantly affect SFCT(29).
Although hypertension is generally associated with reduced CT, the observed increase in SFCT among MACS patients – despite their higher prevalence of hypertension – supports the hypothesis that cortisol's choroidal thickening effect predominates over the thinning influence of hypertension.
In this study, SFCT was significantly greater in the MACS group, whereas CVI did not differ between groups. Based on existing literature, several mechanisms may explain this discrepancy between CT and CVI.
One possible explanation is that increased CT and the presence of pachyvessels in the Haller layer may represent an early phase preceding choriocapillaris ischemia. Beak et al. used OCT-A to analyze the choriocapillaris in eyes with early-stage PSD and found decreased choriocapillaris vascular density corresponding to pachyvessel location in both PPE and UCP eyes compared with controls(30). Similarly, Gal-Or et al. reported a high prevalence of relatively large zones of flow signal attenuation – likely indicative of focal choriocapillaris ischemia – in eyes with pachychoroid disease(31). Kitaya et al. demonstrated reduced foveal choroidal blood flow using laser Doppler flowmetry in eyes with CSCR and suggested that this reduction may correspond to nonperfused areas of the choriocapillaris(32). It can therefore be hypothesized that pachyvessels in the Haller layer may contribute to choriocapillaris ischemia, resulting in increased CT without a corresponding change in CVI. Second, experimental studies have shown that administration of high-dose corticosterone (10 μM) or aldosterone to rat eyes induces choroidal vasodilation and vascular leakage, whereas low-dose glucocorticoids (100 nM) do not elicit such effects(13). In the present study, although patients with MACS showed a slight increase in TCA and LA compared with controls, these differences were not statistically significant. Thus, cortisol levels in MACS – elevated above normal but insufficient to trigger overt vascular remodeling – may have led to an increase in CT without affecting CVI. Accordingly, CT may be a more sensitive parameter than CVI for assessing choroidal involvement in MACS.
To our knowledge, this is the first study to investigate both structural and vascular choroidal changes in patients with MACS. We observed a significant increase in SFCT in this patient group. The main limitations of this study are its cross-sectional design and relatively small sample size.
To minimize the influence of confounding factors, the patient and control groups were carefully matched for axial length, age, and sex – parameters known to affect CT. Furthermore, all OCT scans were performed between 2:00 and 4:00 p.m. to reduce the effect of diurnal variation in CT. Image acquisition was conducted by the same experienced technician, and all measurements were independently evaluated by two observers to enhance the reliability of the findings.
In conclusion, patients with MACS exhibited increased SFCT and a higher frequency of PSD. These findings suggest that MACS may represent an underrecognized risk factor for the development of pachychoroidopathy. Given the observed CT and the potential for associated PSD, routine ophthalmologic evaluation may be warranted in patients with MACS. Future longitudinal studies with larger cohorts are needed to further elucidate the long-term choroidal changes and their implications for visual outcomes in this population.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Ozlem Terzi for her valuable statistical support, which contributed greatly to the success of this study.
We also thank all participants for their time and cooperation.
AUTHORS' CONTRIBUTIONS:
Significant contribution to conception and design: Emrah Kan, Elif KılıçKan, Özlem EksiYucel. Data acquisition: Betül Gündüz, Bilge Eraydın. Data analysis and interpretation: Emrah Kan, Özlem EksiYucel. Manuscript drafting: Emrah Kan. Significant intellectual content revision of the manuscript: Elif KılıcKan, Emrah Kan, Özlem EksiYucel. Final approval of the submitted manuscript: Emrah Kan, Elif KılıçKan, Özlem EksiYucel, Betül Gündüz, Bilge Eraydın. Statistical analysis: Emrah Kan, Özlem EksiYucel. Obtaining funding: not applicable. Supervision of administrative, technical, or material support: Bilge Eraydın., Betül Gündüz. Research group leadership: Elif Kılıç Kan, Emrah Kan.
REFERENCES
1. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144-68.
2. Chung SE, Kang SW, Lee JH, Kim YT. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 2011;118(5):840-5.
3. Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res. 2015;48:82-118.
4. Brown R, Mohan S, Chhablani J. Pachychoroid spectrum disorders: an updated review. J Ophthalmic Vis Res. 2023;19;18(2):212-9.
5. Agrawal R, Gupta P, Tan KA. Cheung CM, Wong TY, Cheng CY. Choroidal vascularity index as a measure of vascular status of the choroid: Measurements in healthy eyes from a population-based study. Sci Rep. 2016;12(6):21090.
6. Haimovici R, Rumelt S, Melby J. Endocrine abnormalities in patients with central serous chorioretinopathy. Ophthalmology. 2003;110(4):698-703.
7. Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997;81(11):962-4.
8. Zhao M, Célérier I, Bousquet E, Jeanny JC, Jonet L, Savoldelli M, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672-9.
9. Nieman LK. Cushing's syndrome: update on signs, symptoms and biochemical screening. Eur J Endocrinol. 2015;173(4):M33-8.
10. Wang E, Chen S, Yang H, Yang J, Li Y, Chen Y. Choroidal thickening and pachychoroid in cushing syndrome: correlation with endogenous cortisol level. Retina. 2019;39(2):408-14.
11. Fassnacht M, Tsagarakis S, Terzolo M, Tabarin A, Sahdev A, Newell-Price J, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2023;189(1):G1-G42.
12. Honda S, Miki A, Kusuhara S, Imai H, Nakamura M. Choroıdal thıckness of central serous chorıoretınopathy secondary to cortıcosteroıd use. Retina. 2017;37(8):1562-7.
13. Zhang CD, Li D, Singh S, Suresh M, Thangamuthu K, Nathani R, et al. Glucocorticoid withdrawal syndrome following surgical remission of endogenous hypercortisolism: a longitudinal observational study. Eur J Endocrinol. 2023;188(7):592-602.
14. Chiodini I, Morelli V, Masserini B, Salcuni AS, Eller-Vainicher C, Viti R, et al. Bone mineral density, prevalence of vertebral fractures, and bone quality in patients with adrenal incidentalomas with and without subclinical hypercortisolism: an Italian multicenter study. J Clin Endocrinol Metab. 2009;94(9):3207-14.
15. Chiodini I, Guglielmi G, Battista C, Carnevale V, Torlontano M, Cammisa M, et al. Spinal volumetric bone mineral density and vertebral fractures in female patients with adrenal incidentalomas: the effects of subclinical hypercortisolism and gonadal status. J Clin Endocrinol Metab. 2004;89(5):2237-41.
16. Soares RR, Samuelson A, Chiang A. Association of chronic central serous chorioretinopathy with subclinical Cushing's syndrome. Am J Ophthalmol Case Rep. 2022;26:101455.
17. Demirel S, Değirmenci MF, Batıoğlu F, Özmert E. Evaluation of the choroidal features in pachychoroid spectrum diseases by optical coherence tomography and optical coherence tomography angiography. Eur J Ophthalmol. 2021;31(1):184-93.
18. Bancos I, Alahdab F, Crowley RK, Chortis V, Delivanis DA, Erickson D, et al. Therapy of endocrine disease: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing's syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175(6):R283-95.
19. Han JM, Hwang JM, Kim JS, Park KH, Woo SJ. Changes in choroidal thickness after systemic administration of high-dose corticosteroids: a pilot study. Invest Ophthalmol Vis Sci. 2014;55(1):440-5.
20. Eymard P, Gerardy M, Bouys L, Mehanna C, Bertherat J, Behar-Cohen F, et al. Choroidal imaging in patients with Cushing syndrome. Acta Ophthalmol. 2021;99(5):533-7.
21. Abalem MF, Machado MC, Santos HN, Garcia R, Helal J Jr, Carricondo PC, et al. Choroidal and retinal abnormalities by optical coherence tomography in endogenous Cushing's syndrome. Front Endocrinol (Lausanne). 2016;7:154.
22. Karaca C, Karaca Z, Kahraman N, Sirakaya E, Oner A, Mirza GE. Is There A Role Of Acth In Increased Choroıdal Thıckness In Cushıng Syndrome? Retina. 2017;37(3):536-43.
23. Holtz JK, Larsson JME, Hansen MS, van Dijk EHC, Subhi Y. Pachychoroid spectrum diseases in patients with cushing's syndrome: a systematic review with meta-analyses. J Clin Med. 2022;11(15):4437.
24. Bouzas EA, Scott MH, Mastorakos G, Chrousos GP, Kaiser-Kupfer MI. Central serous chorioretinopathy in endogenous hypercortisolism. Arch Ophthalmol. 1993;111(9):1229-33.
25. Wei WB, Xu L, Jonas JB, Shao L, Du KF, Wang S, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120(1):175-80.
26. Karahan E, Zengin MO, Aydin R, Ozturk T, Kaya M, Kocak N, et al. Correlation of choroidal thickness with serum cortisol level. Clin Exp Optom. 2015;98(4):362-5.
27. Waghamare SR, Mittal S, Pathania M, Samanta R, Kumawat D, Gupta N, et al. Comparison of choroidal thickness in systemic hypertensive subjects with healthy individuals by spectral domain optical coherence tomography. Indian J Ophthalmol. 2021;69(5):1183-8.
28. Papathanasiou KA, Kazantzis D, Vrachatis DA, Giotaki SG, Papaconstantinou E, Kanakis M, et al. Choroidal thickness in patients with systemic arterial hypertension: a systematic review and meta-analysis. Ther Adv Ophthalmol. 2022 6;14:25158414221132825.
29. Shao L, Zhou LX, Xu L, Wei WB. The relationship between Subfoveal Choroidal Thickness and Hypertensive Retinopathy. Sci Rep. 2021;11(1):5460.
30. Baek J, Kook L, Lee WK. Choriocapillaris Flow Impairments in Association with Pachyvessel in Early Stages of Pachychoroid. Sci Rep. 2019;9(1):5565.
31. Gal-Or O, Dansingani KK, Sebrow D, Dolz-Marco R, Freund KB. Inner choroidal flow signal attenuation in pachychoroid disease: optical coherence tomography angiography. Retina. 2018;38(10): 1984-92.
32. Kitaya N, Nagaoka T, Hikichi T, Sugawara R, Fukui K, Ishiko S, et al. Features of abnormal choroidal circulation in central serous chorioretinopathy. Br J Ophthalmol. 2003;87(6):709-12.
Submitted for publication:
June 25, 2025.
Accepted for publication:
June 25, 2025.
Approved by the following research ethics committee: Ondokuz Mayis University (#B.30.2.ODM.0.20.08/20-28).
Data Availability Statement: The datasets generated and/or analyzed during the current study are included in the manuscript.
Edited by:
Editor-in-Chief: Newton Kara-Júnior Associate Editor: Carlos Augusto Moreira Neto
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: The authors declare no potential conflicts of interest.