

Kenzo Hokazono1; Marília da Cruz Fagundes2; Alessandra Filpo Ferreira da Silva3; Camila Feijó Minku2; Bernardo Corrêa de Almeida Teixeira2; Mário Luiz Ribeiro Monteiro1
DOI: 10.5935/0004-2749.2022-0195
ABSTRACT
A young woman presented at our clinic with sudden visual loss in the right eye, recurrent vertigo, and right-sided tinnitus. We performed a complete ophthalmological evaluation. This revealed effects of the condition on the small arterioles of the peripheral retina. Susac syndrome is characterized by the clinical triad of retinal arteriolar occlusions, cochleovestibular manifestations, and encephalopathy (which can be identified by neuroimaging abnormalities). Early diagnosis and immunosuppressive therapy improved the patient’s visual acuity and the remission of her other symptoms. Hemi-central retinal artery occlusion is an atypical neuro-ophthalmological finding in this disease. However, its identification as a sign of Susac syndrome may facilitate timely diagnosis and accurate treatment.
Keywords: Ophthalmological diagnostic techniques; Retinal artery occlusion; Vertigo; Cerebrovascular disorders; Susac syndrome
INTRODUCTION
Susac syndrome (SS) is a rare autoimmune inflammatory endotheliopathy that causes microangiopathy and leads to vascular occlusions and ischemia in the arterioles of the retina, inner ear, and brain(1).
The clinical triad of branch retinal arterial occlusions (BRAO), hearing loss, and brain involvement (encephalopathy, focal neurological deficits, or headache) is considered pathognomonic(2). However, only 15% of patients present with the complete triad(3).
For diagnosis, typical brain magnetic resonance imaging (MRI) findings are also required. These include fluid-attenuated inversion recovery (FLAIR) and T2 hyperintense, small, round multifocal snowball-like lesions of the white matter, at least one of which is in the corpus callosum. Leptomeningeal enhancement may also be observed.
CASE REPORT
A 27-year-old woman presented with a sudden onset of visual loss in the right eye (OD). She also complained of recurrent episodes of vertigo, right-sided tinnitus, and auricular plenitude sensations over the previous week.
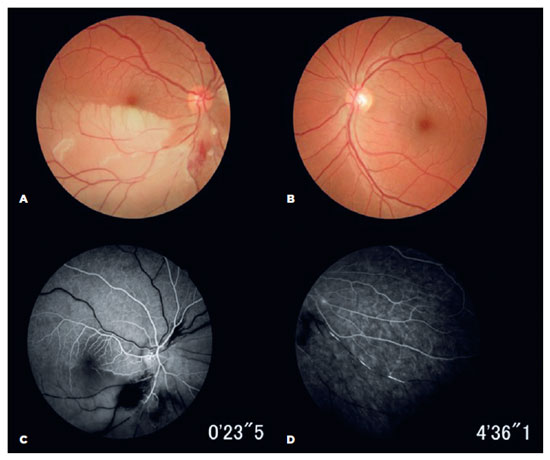
Ophthalmic examination revealed a best-corrected visual acuity of 20/20 in both eyes (OU). Fundus examination identified a hemi-central retinal artery occlusion (CRAO) in the OD. Fundus fluorescein angiography (FA) confirmed an ipsilateral occlusion of the inferior arterial hemi-trunk and hyper-fluorescence was observed in the arteriolar vessel walls, with multiple smaller BRAOs in the OU (Figure 1).
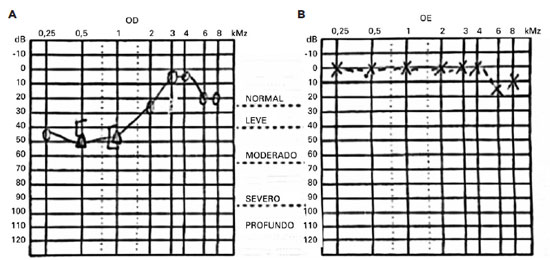
A Rinne test yielded positive results on both sides, and a Weber test lateralized to the left ear, suggesting sensorineural hearing loss. An audiogram confirmed low-frequency sensorineural hearing loss in the right ear (Figure 2).
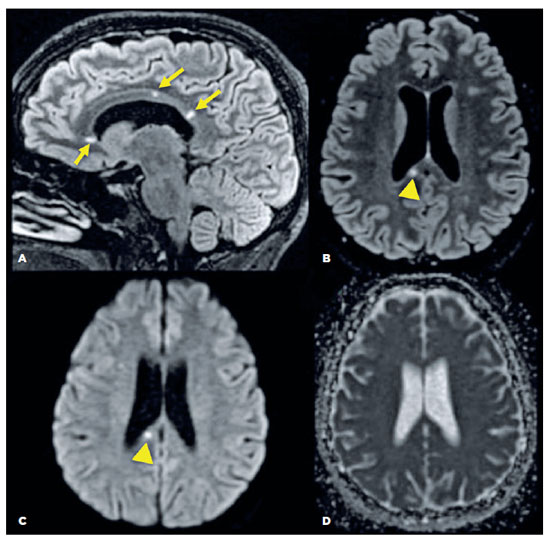
A brain MRI of the patient revealed small spherical high-signal intensity lesions on FLAIR and T2 sequences. These were located within the fiber tracts of the corpus callosum, with no ependymal undersurface involvement. The dominant lesion in the splenium showed restricted diffusion indicative of a small ischemic infarct (Figure 3).
A workup was performed to exclude vascular and embolic diseases. This included complete blood count; erythrocyte sedimentation rate; levels of C-reactive protein, antinuclear antibodies, serum complement, IgG and IgM anticardiolipin antibodies, and lupus anticoagulant. All of these were within the normal range. The results of an analysis of the cerebrospinal fluid, a transthoracic echocardiogram, and a Doppler ultrasound of cervical arteries were all unremarkable.
The presence of the clinical triad of multiple retinal artery occlusion, cochleovestibular involvement, and brain abnormalities, confirmed by MRI established the diagnosis as SS.
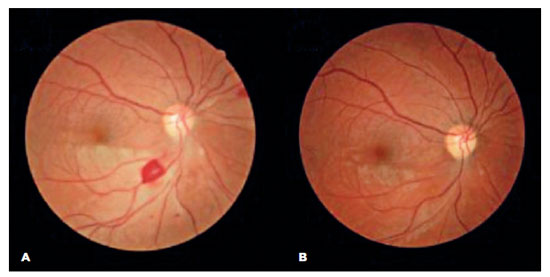
The patient was treated with endovenous high dose methylprednisolone and discharged with oral azathioprine and a prednisone tapering plan. At her two-month follow-up visit, she reported an improvement in her visual acuity and no additional symptoms. A fundus examination revealed a reduction in retinal edema and opacity in the OD and partial ipsilateral revascularization (Figure 4A), which was no longer apparent at the next follow-up (Figure 4B).
DISCUSSION
In 1979, Susac et al.(1) described a syndrome involving microangiopathy of the brain and retina. The authors reported two cases, both young women, who presented with psychosis, hearing loss, and multiple BRAO. Subsequently, Coppeto et al.(4) and Monteiro et al.(2) reported four more cases, allowing a comprehensive characterization of the syndrome as a distinct entity. In these reports, the authors highlighted BRAO as a clinical feature of SS, which was present in both eyes in five of the six reported cases. Since then, other case reports and case series have been published, emphasizing BRAO as a key clinical feature of SS.
Nowadays, SS is classically associated with impairment of the peripheral retina, causing multiple recurrent BRAO and yellow, non-refractile or refractile, retinal arterial wall plaques (Gass plaques). These are found in mid-arteriolar segments visible as fluorescence on fundus FA(5). It is likely that the endothelial injury in SS causes turbulent flow, leading to fibrin-like white material deposits in the vessel wall, (which is different from platelet emboli, which accumulate at the bifurcation)(2), predisposing it to in situ thrombosis(6). Therefore, to detect this peripheral retinal microangiopathy, a comprehensive fundus examination of both eyes, including FA, should be performed. Attention should be paid to any damage to small arterioles. This should be accompanied by further otological evaluation and careful neuroimaging analysis.
As SS usually presents with a preference for peripheral retinal arterioles, only a few cases have been reported in which the central retinal artery or its first branch was initially occluded(2). Hemi-CRAO is very unusual in SS and was the presenting sign in our case, making it unique.
Adatia et al.(7) have described the case of a 36-year-old woman who presented with CRAO one month after the onset of neurological symptoms, including episodic blurred speech, decreased hearing, paresthesia, and weakness. Apostolos-Pereira et al.(5) have reported a case of a 24-year-old man with CRAO in the left eye as the sole presenting sign of SS. Three weeks later, the patient developed hearing loss, encephalopathy and multiple BRAO in the right eye.
Buelens et al.(8) recently published the case of a 22-year-old woman with CRAO in one eye and a negative comprehensive workup. After ruling out all neurological diseases and treating the patient empirically, an audiogram was performed, revealing mild sensorineural hearing loss in the left ear. Two weeks later, a repeat MRI showed multiple periventricular hyperintense lesions in the white matter, with corpus callosum involvement, which was highly indicative of SS.
The diagnostic workup of CRAO involves the exclusion of thromboembolic causes. Accordingly, inflammatory and coagulation disorders must also be considered, especially with a unilateral hemi-CRAO. Although emboli and coagulopathy are the most common causes of CRAO, the above cases demonstrate that SS should be included in the differential diagnosis, particularly in younger and healthy patients with this ophthalmic presentation, as the SS clinical triad does not usually present simultaneously(4). Patients with CRAO should undergo a careful examination of the peripheral retina for multiple BRAO.
In conclusion, hemi-CRAO can be considered an atypical neuro-ophthalmological finding related to this disorder. Its presence should increase the suspicion of SS, facilitating its timely diagnosis. Considering the presumed autoimmune etiology of SS, accurate treatment with immunosuppression is necessary. This can improve a patient’s visual acuity and eradicate other symptoms(6).
ACKNOWLEDGMENTS
The authors acknowledge the patient for her generous cooperation.
REFERENCES
1. Susac JO, Hardman JM, Selhorst JB. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313-6.
2. Monteiro ML, Swanson RA, Coppeto JR, Cuneo RA, DeArmond SJ, Prusiner SB. A microangiopathic syndrome of encephalopathy, hearing loss, and retinal arteriolar occlusions. Neurology. 1985;35(8):1113-21.
3. Kleffner I, Dörr J, Ringelstein M, Gross CC, Böckenfeld Y, Schwindt W, et al. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87(12):1287-95.
4. Coppeto JR, Currie JN, Monteiro ML, Lessell S. A syndrome of arterial-occlusive retinopathy and encephalopathy. Am J Ophthalmol. 1984;98(2):189-202.
5. Apostolos-Pereira SL, Kara-Jose LB, Marchiori PE, Monteiro ML. Unilateral central retinal artery occlusion as the sole presenting sign of Susac syndrome in a young man: case report. Arq Bras Oftalmol. 2013;76(3):192-4.
6. Pereira S, Vieira B, Maio T, Moreira J, Sampaio F. Susac’s syndrome: An updated review. Neuroophthalmology. 2020;44(6):355-60.
7. Adatia FA, Sheidow TG. Central retinal artery occlusion as the initial ophthalmic presentation of Susac’s syndrome. Can J Ophthalmol. 2004;39(3):288-91.
8. Buelens T, Herode L, Nubourgh I, Caspers L, Willermain F, Postelmans L. Central retinal artery occlusion and Susac syndrome: a case report. Retin Cases Brief Rep. 2014;8(3):187-92.
Submitted for publication:
August 9, 2022.
Accepted for publication:
February 2, 2024.
Approved by the following research ethics committee: Hospital de Clínicas da Universidade Federal do Paraná (CAAE: 59792122.7.0000.0096).
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.