

Deise F. Costa1,2; Carmen Luz Pessuti2; Thupten Tsering1; Mohamed Abdouh1; Kleber Ribeiro2; Heloisa Nascimento2; Alessandra G. Commodaro2; Julia V. Burnier3; Rubens Belfort Jr2; Miguel N. Burnier Jr1
DOI: 10.5935/0004-2749.2023-0037
ABSTRACT
PURPOSE: To characterize the extracellular vesicle protein cargo in the aqueous humor and plasma of patients with ocular toxoplasmosis.
METHODS: Aqueous humor and plasma were collected from six patients with active ocular toxoplasmosis and six patients with cataract. Extracellular vesicles were isolated, and western blotting and mass spectrometry were performed for protein analysis.
RESULTS: All plasma samples from patients with ocular toxoplasmosis and cataract were positive for the tetraspanins CD63 and TSG101. However, the aqueous humor from patients with ocular toxoplasmosis was positive only for CD63. Sixty-seven new unreported proteins were identified in the aqueous humor and plasma of patients with the ocular toxoplasmosis and cataract. Of the 67 proteins, 10 and 7 were found only in the cataract and ocular toxoplasmosis groups, respectively. In general, these proteins were involved in immune system activation and retina homeostasis and were related to infections and retina-associated diseases. Conclusion: The distinct protein signatures between ocular toxoplasmosis and cataract may be helpful in the differential diagnosis of ocular toxoplasmosis. However, more studies are needed to better understand the role of these proteins in the pathogenesis of ocular toxoplasmosis.
Keywords: Extracellular vesicles; Proteomics; Toxoplasma gondii; Ocular toxoplasmosis, Aqueous humor; Plasma; Liquid biopsy
INTRODUCTION
Ocular toxoplasmosis (OT) is caused by the protozoan parasite Toxoplasma gondii (T. gondii), and it can be congenital or acquired postnatally(1). The parasite infects approximately 25%-30% of the human population worldwide(2). However, the seroprevalence varies between different geographic areas and countries(3). In Brazil, up to 50% of elementary school children and 50%–80% of women of childbearing age demonstrate antibodies against T. gondii. Thus, the seroprevalence in Brazil is four times higher than that in USA(4).
Ocular manifestations of OT include floaters and blurred vision. Additionally, decreased visual acuity occur because of macular involvement, optic atrophy, or severe vitreous inflammation(5).
Accurate diagnosis depends on the clinical features of the disease. However, atypical presentations and resemblance to other lesions (e.g., fungal retinitis, septic retinitis, and ocular toxocariasis) are sources of diagnostic challenges, which often lead to misdiagnosis and inappropriate treatment(5).
Similar to several cell types, parasites release extracellular vesicles (EVs) that are postulated to be involved in cell-cell communication or in the modulation of host immune responses(6). The EVs typically consist of a lipid bilayer membrane, containing integral membrane proteins, and a luminal cavity loaded with a variety of soluble proteins and nucleic acids (RNA and DNA)(6). The EVs present a certain set of molecules that include proteins such as tumor susceptibility gene 101 (TGS101), CD9, and CD63; some of these proteins are considered essential components of the EVs. These EV marker proteins have been used to confirm the presence of EVs via immunoblotting(7). Additionally, EVs serve as biomarkers for various diseases, including melanoma(8) and epithelial ovarium cancer(9).
Exosomes have been identified in aqueous humor (AH)(10) and vitreous samples(11). A study revealed that specific proteins were elevated in the AH of individuals with age-related macular degeneration (AMD). These results indicate that exosomal proteins in AH may be used as biomarkers for the diagnosis of AMD(12).
EVs isolated from biological fluids could be a useful tool for the early diagnosis and treatment of OT. Thus, herein, we aimed to characterize the EV protein cargo in the AH and plasma of patients with OT.
METHODS
Patients
Six patients with active OT and six patients with a cataract (CAT) were recruited from March to July 2018 at the Department of Ophthalmology, UNIFESP/EPM, Brazil and Vision Institute, IPEPO, Brazil. Six patients diagnosed with active OT (4 women and 2 men; average age, 39 years) who tested seropositive for T. gondii underwent an ocular examination, including measurement of best-corrected visual acuity, applanation tonometry, undilated and dilated slit-lamp biomicroscopy, and indirect fundus examination. All six patients demonstrated more than 1+ cells in the anterior chamber, unilateral (n=5) or bilateral (n=1) involvement, recurrence (n=4) or no recurrence (n=2), and OT lesions characterized by typical focal necrotizing retinochoroiditis (Table 1). The six patients with a CAT who underwent routine cataract surgery (2 women and 4 men; average age, 68 years) were included as controls.
This study was approved by the institutional ethics committee (No: 2198149) and was conducted in adherence to the principles of the Declaration of Helsinki and Resolution 196/96 of the Ministry of Health, Brazil. Informed consent was obtained from all the participants.
Aqueous humor and plasma sample collection
Eleven peripheral blood and 12 AH samples were collected from the 12 patients. Peripheral blood (10 mL) was collected in EDTA tubes and centrifuged for 10 min at 1900 × g. Subsequently, 1 mL aliquots of plasma were collected. Anterior chamber paracentesis was performed using a slit lamp after application of a topical anesthetic and topical antiseptic. The ophthalmologist used a 1 mL tuberculin syringe (27-gauge ½-inch needle) to aspirate 0.1 mL of the AH. All samples were stored at 4°C until the assays were performed.
EV isolation
The EVs were isolated according to the guidelines of the International Society for Extracellular Vesicles (ISEV-2018). The samples (1 mL plasma or 100 µl AH) were centrifuged at 16,000 × g for 10 min at 4°C to eliminate cellular debris. Thereafter, the EVs were isolated using the exoEasy Maxi Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. The samples were gently mixed with the same volume of buffer XBP. The mixture was charged onto the exoEasy spin column and centrifuged at 500 × g for 1 min. After washing the column with buffer XWP, the EVs were eluted in 250 µl of buffer XE by centrifugation at 500 × g for 5 min.
EV characterization
Aliquots of isolated EVs were diluted 100 times in PBS and processed using a nanoparticle tracking analysis (NTA) system and the NanosightNS300 (Malvern, UK). The samples were read in triplicate for 30 s at 20 frames/s. NTA (version 3.2) was used to estimate the concentration and size of the EV particles isolated from both the plasma and AH.
The EVs were lysed in RIPA buffer containing complete mini protease inhibitors (Sigma) at 4°C for 30 min. The samples were pulse sonicated for 2 s (3 times) and centrifuged at 13,000 x g for 30 min at 4°C. Protein concentrations were quantified using the BCA assay (Thermo Fisher Scientific) according to the manufacturer’s instructions. Protein samples were processed for western blotting and mass spectrometry (MS) proteomic analysis.
Western blotting
Aliquots of 20 µg of EV proteins were separated using 12% precast polyacrylamide gel (Mini-PROTEAN; BioRad) in running buffer at 120 V for 70 min. The proteins were electrotransferred onto polyvinylidene difluoride (PVDF) membranes using Trans-Bot Turbo transfer (Biorad). Subsequently, the PVDF membranes were blocked for 1 h at room temperature with 5% non-fat dry milk in 1X Tris-buffer saline with 0.05% Tween 20. The membranes were probed with primary antibodies against TSG101, CD63 (Abcam 1:1000), or GM-130 (Abcam 1:1000), followed by HRP-conjugated secondary antibodies [goat anti-rabbit (Sigma 1:1000) or goat anti-mouse (Sigma 1:3000)]. The membranes were washed five times for 10 min each after each incubation, developed using the ECL prime Western blot detection reagent (GE healthcare), and visualized using the ChemiDocTM XRS+ System.
Transmission electron microscopy
Carbon-coated copper transmission electron microscopy (TEM) grids were negatively charged using PELCO easiGlow, and 20 µl of the EV sample was layered on it for 20 min. Following sample adsorption, the grids were quickly and gently washed with milliQ water for 10 min. Excess water was removed using a filter paper. It was immediately negatively stained with 2% uranyl acetate for 4 min, and dried on the filter paper. Imaging was performed using a 120 kV Cryo-transmission electron microscope (FEI Tecnai G2 Spirit Twin; ) and a camera system (Ultrascan 4000 4k × 4k CCD Model 895; Gatan).
Label-free liquid chromatography-MS/MS proteomics analysis of EVs and database search
Aliquots of 20 μg of EV proteins from each sample were loaded onto a single stacking gel band to eliminate contaminants such as lipids, detergents, and salts. Each sample was run in duplicate.
The gel band was reduced with dithiothreitol (DTT), alkylated with iodoacetic acid, and digested with trypsin. The extracted peptides were resolubilized in 0.1% aqueous formic acid and loaded onto an Acclaim PepMap (75 μm inner diameter × 2 cm, C18 3 μm particle size; Thermo Scientific) precolumn. Subsequently, they were loaded onto an Acclaim PepMap EASY-Spray (75 μm inner diameter × 15 cm, 2 μm C18, 2 μm beads) analytical separation column using a Dionex UltiMate 3000 uHPLC at 250 nL/min, with a gradient of 2%-35% organic (0.1% formic acid in acetonitrile) over 3 h. The peptides were analyzed using an Orbitrap Fusion MS (Thermo) operating at 120,000 resolution (full width at half maximum in MS1) with higher-energy collisional dissociation sequencing (15,000 resolution) at top speed for all peptides and a charge of ≥2+. The MS raw data were converted into *.mgf format (Mascot generic format) and searched using the Mascot search engine (version 2.6.2; Matrix Science) against human protein sequences (Uniprot 2019). The database search results were loaded onto Scaffold Q+ Scaffold (version 4.10.0; Proteome Sciences) for spectral counting, statistical treatment, data visualization, and quantification. Protein threshold >99%, peptide threshold >95%, and two of a minimum number of unique peptides were applied in Scaffold Q+ to increase the confidence level of identified proteins. Additional filters such as a p-value of <0.05 and a fold-value change of ≥2 were used to identify the differential expression of proteins. The identified protein list in Scaffold was exported to Microsoft Excel and uploaded into the DAVID bioinformatics database (version 6.8) for gene ontology analyses (i.e., biological process, cellular component, and KEGG pathway). Additionally, bioinformatic analysis and a Vesiclepedia database search were performed using FunRich (version 3.1.3).
The MS proteomics data have been deposited in the ProteomeXchange Consortium via the PRIDE partner repository (dataset identifier: PXD046167).
RESULTS
Proteomic analysis of EVs isolated from plasma and AH samples
All five plasma-derived EV samples from the patients with OT were positive for TSG101 and CD63 markers; however, 4 of 6 AH-derived EV samples were positive only for CD63 (Figure 1A). All six plasma-derived EV samples from the patients with CAT were positive for the CD63 marker; however, all the AH-derived EV samples were negative for both markers (Figure 1A). All the analyzed EV samples were negative for GM-130 (a negative EV marker), indicating that the EV preparations were pure and not contaminated with other cellular organelles (Figure 1A). To obtain more insight into the identity of the isolated particles, we physically analyzed them using NTA and TEM. The isolated EVs demonstrated a standard distribution pattern when analyzed by NTA (Figure 1B–E). They had a mean size of 204–290 nm, with no difference based on their origin (plasma vs. AH) or patient’s health status (OT vs. CAT). TEM analyses demonstrated that the isolated particles were rounded structures ranging from 100 to 250 nm in size (Figure 1F–H). These findings are similar to those of our previous studies(13,14), indicating that the analyzed particles demonstrated the phenotypic and physical characteristics of EVs.
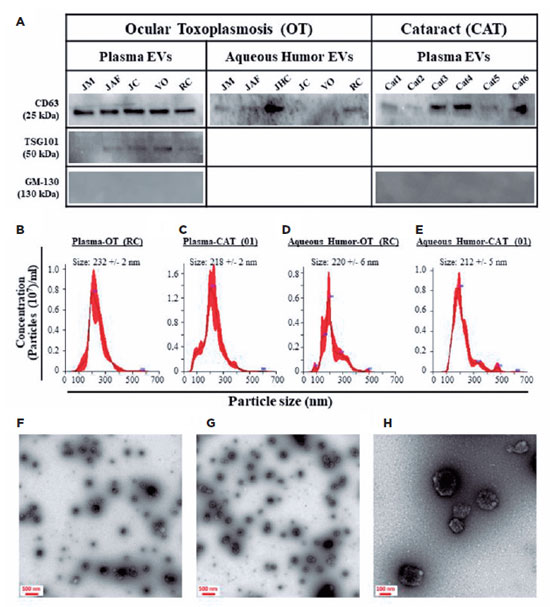
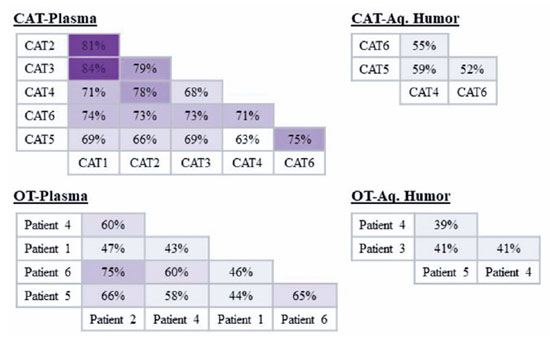
For protein profile analysis, the EVs were isolated in patients with OT (AH, n=3; plasma n=5) and patients with CAT (AH, n=3; plasma n=6) (Table 1). In the OT group, protein similarities in EVs varied from 43% (Patient 1 vs. Patient 4) to 75% (Patient 6 vs. Patient 2) in the plasma and from 39% (Patient 4 vs. Patient 5) to 41% (Patient 3 vs. Patient 5 and Patient 3 vs. Patient 4) in the AH. In the control group, similarities ranged from 63% (CAT 5 vs. CAT 4) to 84% (CAT 3 vs. CAT 1) in the plasma and from 52% (CAT 5 vs. CAT 6) to 59% (CAT 5 vs. CAT 4) in AH (Figure 2). These results indicate that the protein cargo from EVs was consistent among the samples analyzed in this study.
A total of 803 proteins were identified in the AH and plasma from both the OT and CAT groups (Table 2). Among these, 736 (92%) had already been reported in the Vesiclepedia database, as determined by the FunRich bioinformatics analysis (Figure 3A).

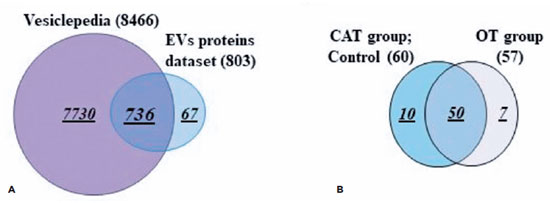
Figure 3. Proteomic minning of the EV cargo revealed the presence of new proteins. (A) Venn diagram analyses demonstrate that the majority (90–93%) of the proteins isolated from EVs derived from different samples were published in the Vesiclepedia database. Note: Sixty-seven proteins were not previously reported. (B) Venn diagram showing the distribution of the newly reported EV proteins in the different groups (CAT vs. OT).
Sixty-seven proteins were not previously reported and have been described here in association with EVs for the first time (Figure 3A and Table 3). Among these 67 proteins, 50 were found in both OT and CAT groups, 10 were found only in the CAT group, and seven were found only in the OT group (Figure 3B). Additionally, among the seven proteins in the OT group, two were described only in the AH in patient 3 (Table 2). The other five proteins were found in the plasma. The protein ARHGAP45 was detected in patients 2, 4, 5, and 6, the ACAN protein was detected in patients 1, 2, and 4, and the ERBIN protein was detected in patients 2, 5, and 6 (Table 2).
OT sample-derived EVs were enriched in proteins related to eye diseases
EV proteins from the plasma and AH of patients with OT were clustered to identify their relation to physiological processes (Table 4). Cluster analysis by cellular component demonstrated sets of proteins related to extracellular exosomes, microparticles, vesicles, and endosomes (Figure 4A). Moreover, the analysis highlighted categories consistent with pathways related to complement activation, immune response activation, and retinal homeostasis (Figure 4B).
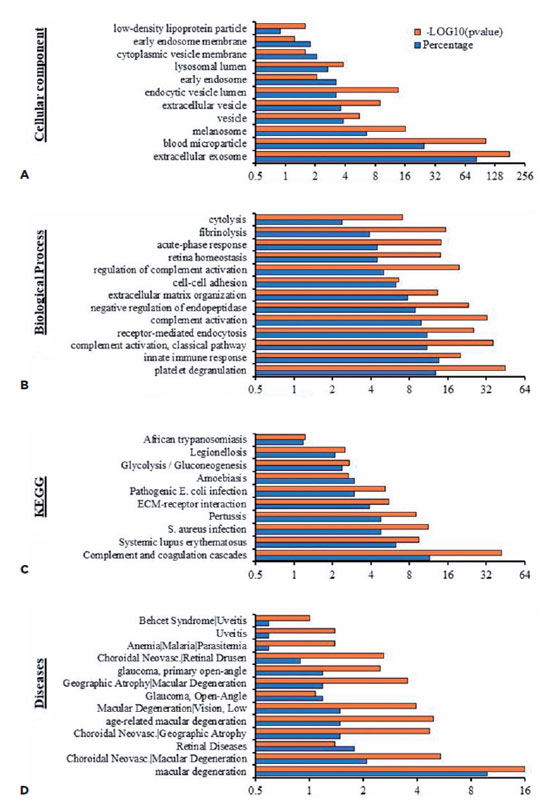
Molecular function clustering using KEGG pathway analysis revealed that isolated EVs were related to different infectious diseases, including African trypanosomiasis, Legionellosis, amoebiasis, and pathogenic E. coli (Figure 4C). In addition, analysis for associated diseases demonstrated that the proteins were clustered in retinal-associated diseases such as uveitis, choroidal neovascularization, and macular degeneration (Figure 4D).
DISCUSSION
Classic early symptoms of OT are similar to those of uveitis in general, such as pain, redness, photophobia, and decreased vision(5). Systemic protein biomarkers or biomarkers in the ocular fluids of patients with uveitis may help with diagnosis, prognosis, and treatment. Herein, we aimed to characterize the EV protein cargo isolated from the AH and plasma of patients with OT and compare it with that of patients with cataract (control group).
Several cells secrete EVs under physiological and pathological conditions(6). EVs have been identified in the tears(15), aqueous humor(10, 13), vitreous humor(16), and blood(17) of patients with several ocular diseases(16,17). The proteins CD63 and TSG101 are common biochemical constituents in all EV subtypes(7). Recent studies have used CD63 and TSG101 as markers to identify and characterize EVs in patients with lung cancer(18), uveal melanoma(14), chronic Chagas disease(19) and cerebral toxoplasmosis(20). In this study, we analyzed the expression of these EV markers and found that all the plasma samples from patients with OT and CAT were positive for both CD63 and TSG101. Four of the six AH samples from patients with OT were positive only for CD63. However, all the AH samples from patients with CAT were negative for both markers. Thus, the EV marker profiles appear to differ between the plasma and AH, and CD63-positive EVs are more commonly found in the AH of patients with OT than in the AH of the controls. This may be associated with the pathological conditions in the eye caused by T. gondii.
Our proteomic analysis detected 803 proteins differentially expressed in the plasma and AH of patients with OT and CAT. Among these, we identified 67 new proteins that have not been previously reported. Of these 67 proteins, seven were only detected in patients with OT, and 10 were detected only in patients with CAT.
In general, the proteins differentially expressed in the AH or plasma samples from patients with OT cover a diverse spectrum of functions associated with inflammatory processes commonly encountered in OT. For instance, the integrin metalloprotease ADAMDEC1 is primarily expressed in myeloid lineage cells and is upregulated by various stimuli and inflammatory states(21). The overexpression of ADAMDEC1 has been described in cutaneous disease, pulmonary sarcoidosis, and systemic lupus erythematosus(22). Fc receptor-like 5 is a novel IgG-binding protein expressed on B cells, and it is involved in the pathogenesis of inflammatory and infectious diseases(23). This protein was described in AH in patients with OT for the first time in this study. However, a study with a larger sample size is required to confirm that the protein can be used as a biomarker.
Among the proteins differentially expressed in the plasma of patients with OT, we identified the Aggrecan core protein (PGCA), a proteoglycan that is the main component of the extracellular matrix of cartilaginous tissue(24). We also found soluble guanylyl cyclase (sGC), a heterodimeric enzyme that is a crucial intracellular target of the signaling molecule nitric oxide (NO)(25). High levels of NO have been identified in human Muller cells and the retinal pigment epithelium in response to cytomegalovirus infection(26). In OT, NO plays a crucial role in protecting against T. gondii infection(27).
Another highlighted protein was ArhGAP45, which acts as a Rac-GAP (GTPase-Activating Protein) in endothelial cells. It has a negative effect on endothelial barrier function. Silencing ArhGAP45 promotes basal endothelial barrier functions, whereas the loss of ArhGAP45 promotes migration and shear stress adaptation. This suggests that ArhGAP45 is a novel regulator that fine-tunes the regulation of basal endothelial integrity(28). Erbin reportedly plays an important role in cell polarization, receptor localization, and signal transduction(29). It is reportedly involved in the activation of NF-κB and cytokine secretion by interacting with Nod2(30).
Proteomic mining of isolated EVs from the plasma and AH of patients with OT revealed a set of proteins involved in immune system activation, infectious diseases, retina homeostasis, and retina-associated diseases (i.e., uveitis and macular degeneration). These protein profiles warrant further investigation in relation to OT.
The label-free LC-MS used in our study has its limitations that it shares with methods that rely on quantification based on peptide ion peak area measurement. These issues include chromatographic alignment, peptide qualification for quantitation, and normalization. Nonetheless, it remains a viable alternative to array-based, gel-based, and stable isotope tag- or label-based approaches. Our analyses reveal that comprehensive, accurate, and reproducible protein identification and quantification are achievable between all sample groups.
In conclusion, EV-derived proteins from the plasma and/ or AH of patients with OT could be used as biomarkers in the future. The role of these proteins in the pathogenesis of OT needs further investigation, which may lead to the development of new treatment and diagnostic strategies.
ACKNOWLEDGMENTS
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (RBJ: CNPq process No. 429571/2018-6), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). RBJ is a recipient of CNPq fellowships.
REFERENCES
1. Gilbert RE, Stanford MR. Is ocular toxoplasmosis caused by prenatal or postnatal infection? Br J Ophthalmol. 2000;84(2):224-6.
2. Maenz M, Schlüter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res. 2014;39:77-106.
3. Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009; 39(12):1385-94.
4. Dubey JP. Toxoplasmosis of Animals and Humans. Boca Raton: CRC Press; 2010.
5. da Mata AP, Orifice F. Toxoplasmosis. In: Foster CS, Vitale AT. Diagnosis and treatment of uveitis. Philadelphia: WB Saunders; 2002. p. 385-410.
6. Trocoli Torrecilhas AC, Tonelli RR, Pavanelli WR, da Silva JS, Schumacher RI, de Souza W, et al. Trypanosoma cruzi: parasite shed vesicles increase heart parasitism and generate an intense inflammatory response. Microbes Infect. 2009;11(1):29-39.
7. Bobrie A, Colombo M, Krumeich S, Raposo G, Théry C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1:18397.
8. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883-91.
9. Zhang W, Ou X, Wu X. Proteomics profiling of plasma exosomes in epithelial ovarian cancer: A potential role in the coagulation cascade, diagnosis and prognosis. Int J Oncol. 2019;54(5):1719-33.
10. Dismuke WM, Challa P, Navarro I, Stamer WD, Liu Y. Human aqueous humor exosomes. Exp Eye Res. 2015;132:73-7.
11. Zhao Y, Weber SR, Lease J, Russo M, Siedlecki CA, Xu LC, et al. Liquid Biopsy of Vitreous Reveals an Abundant Vesicle Population Consistent With the rSize and Morphology of Exosomes. Transl Vis Sci Technol. 2018;7(3):6.
12. Kang GY, Bang JY, Choi AJ, Yoon J, Lee WC, Choi S, et al. Exosomal proteins in the aqueous humor as novel biomarkers in patients with neovascular age-related macular degeneration. J Proteome Res. 2014;13(2):581-95.
13. Pessuti CL, Costa DF, Ribeiro KS, Nascimento H, Belfort R, Commodaro AG, et al. Extracellular vesicles from the aqueous humor of patients with uveitis. Pan Am J Ophthalmol 2019;1(1):1-3.
14. Pessuti CL, Costa DF, Ribeiro KS, Abdouh M, Tsering T, Nascimento H, et al. Characterization of extracellular vesicles isolated from different liquid biopsies of uveal melanoma patients. J Circ Biomark. 2022;11(1):36-47.
15. Grigor’eva AE, Tamkovich SN, Eremina AV, Tupikin AE, Kabilov MR, Chernykh VV, et al. [Characteristics of exosomes andmicroparticles discovered in human tears]. Biomed Khim. 2016;62(1):99-106. Russian.
16. Ragusa M, Barbagallo C, Statello L, Caltabiano R, Russo A, Puzzo L, et al. miRNA profiling in vitreous humor, vitreal exosomes and serum from uveal melanoma patients: Pathological and diagnostic implications. Cancer Biol Ther. 2015;16(9):1387-96.
17. Huang C, Fisher KP, Hammer SS, Navitskaya S, Blanchard GJ, Busik JV. Plasma exosomes contribute to microvascular damage in diabetic retinopathy by activating the classical complement pathway. Diabetes. 2018;67(8):1639-49.
18. Novikova SE, Soloveva NA, Farafonova TE, Tikhonova OV, Liao PC, Zgoda VG. Proteomic signature of extracellular vesicles for lung cancer recognition. Molecules. 2021;26(20):6145.
19. Madeira RP, Meneghetti P, de Barros LA, de Cassia Buck P, Mady C, Ianni BM, et al. Isolation and molecular characterization of circulating extracellular vesicles from blood of chronic Chagas disease patients. Cell Biol Int. 2022;46(6):883-94.
20. da Cruz AB, Maia MM, Pereira IS, Taniwaki NN, Namiyama GM, Telles JP, et al. Human extracellular vesicles and correlation with two clinical forms of toxoplasmosis. PLOS ONE. 2020;15(3):e0229602.
21. Galamb O, Györffy B, Sipos F, Spisák S, Németh AM, Miheller P, et al. Inflammation, adenoma and cancer: objective classification of colon biopsy specimens with gene expression signature. Dis Markers. 2008;25(1):1-16.
22. Crouser ED, Culver DA, Knox KS, Julian MW, Shao G, Abraham S, et al. Gene expression profiling identifies MMP-12 and ADAMDEC1 as potential pathogenic mediators of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2009;179(10):929-38.
23. Li H, Borrego F, Nagata S, Tolnay M. Fc Receptor-like 5 expression distinguishes two distinct subsets of human circulating tissue-like memory B cells. J Immunol. 2016;196(10):4064-74.
24. Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell Res. 2002;12(1):19-32.
25. Marro ML, Peiró C, Panayiotou CM, Baliga RS, Meurer S, Schmidt HH, et al. Characterization of the human alpha1 beta1 soluble guanylyl cyclase promoter: key role for NF-kappaB (p50) and CCAAT-binding factors in regulating expression of the nitric oxide receptor. J Biol Chem. 2008;283(29):20027-36.
26. Dighiero P, Reux I, Hauw JJ, Fillet AM, Courtois Y, Goureau O. Expression of inducible nitric oxide synthase in cytomegalovirus-infected glial cells of retinas from AIDS patients. Neurosci Lett. 1994;166(1):31-4.
27. Hayashi S, Chan CC, Gazzinelli RT, Pham NT, Cheung MK, Roberge FG. Protective role of nitric oxide in ocular toxoplasmosis. Br J Ophthalmol. 1996;80(7):644-8.
28. Amado-Azevedo J, Reinhard NR, van Bezu J, van Nieuw Amerongen GP, van Hinsbergh VWM, Hordijk PL. The minor histocompatibility antigen 1 (HMHA1)/ArhGAP45 is a RacGAP and a novel regulator of endothelial integrity. Vasc Pharmacol. 2018;101:38-47.
29. Yao S, Zheng P, Wu H, Song LM, Ying XF, Xing C, et al. Erbin interacts with c-Cbl and promotes tumourigenesis and tumour growth in colorectal cancer by preventing c-Cbl-mediated ubiquitination and down-regulation of EGFR. J Pathol. 2015;236(1):65-77.
30. McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lécine P, et al. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem. 2005;280(48):40301-9.
Submitted for publication:
February 7, 2023.
Accepted for publication:
December 13, 2023.
Approved by the following research ethics committee: Hospital São Paulo – Hospital Universitário da UNIFESP (CAAE: 62823616.2.0000.5505).
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.