

Fábio Ejzenbaum1; Tania Mara Cunha Schaefer2; Celso Cunha3; Julia Dutra Rossetto4; Izabela F. Godinho5; Célia Regina Nakanami6; Regina K. Noma7; Luisa Moreira Hopker8
DOI: 10.5935/0004-2749.2023-0009
ABSTRACT
This document on myopia control is derived from a compilation of medical literature and the collective clinical expertise of an expert committee comprising members from the Brazilian Society of Pediatric Ophthalmology and the Brazilian Society of Contact Lenses and Cornea. To manage myopia in children, the committee recommends corneal topography and biannual visits with cycloplegic refraction, along with annual optical biometry. For fast-progressing myopia, biannual biometry should be considered. Myopic progression is defined as an annual increase in spherical equivalent greater than 0.50 D/year or in axial length greater than 0.3 mm (until 10 years old) or 0.2 mm (above 11 years). The proposed treatments for myopia progression include environmental control, low concentration atropine, defocus glasses, contact lenses, or Ortho-K lenses, and combinations of these methods may be necessary for uncontrolled cases. Treatment should be sustained for at least 2 years. This document serves as a comprehensive guideline for diagnosing, treating, and monitoring pre-myopic and myopic children in Brazil.
Keywords: Myopia; Pupil disorders; Disease progression; Atropine; Refraction; Ocular; Mydriatics; Contact lens; Biometry; Child; Brazil
RESUMO
Esta revisão foi baseada na literatura médica e na experiência clínica de um comitê de especialistas membros da Sociedade Brasileira de Oftalmologia Pediátrica e da Sociedade Brasileira de Lentes de Contato e Córnea. Rotineiramente as crianças devem ser submetidas a topografia da córnea no primeiro exame e visitas semestrais com refração cicloplegiada e biometria óptica anual. A progressão da miopia foi definida como um aumento anual no equivalente esférico maior que 0,50 D/ano ou do comprimento axial maior que 0,3 mm (até 10 anos) ou 0,2 mm (mais de 11 anos). Os tratamentos propostos para a progressão são controle ambiental, atropina em baixa concentração, óculos com defocus, lentes de contato ou ortoceratologia, devendo-se considerar associações para casos não controlados. O tratamento deve ser realizado por pelo menos 2 anos. O presente documento é uma diretriz para diagnóstico, tratamento e acompanhamento de crianças pré-míopes e míopes no Brasil.
Descritores: Miopia; Distúrbios pupilares; Progressão da doença; Atropina; Refração ocular; Midriáticos; Lentes de contato; Biometria; Criança; Brasil
INTRODUCTION
Uncorrected myopia represents a significant cause of preventable visual impairment, having potential impacts on children’s learning, school performance, and self-esteem(1,2). Studies conducted on samples of campaigns and collective efforts among school-age children in Brazil have indicated an overall prevalence ranging from 2.1% to 13.3%. Despite an increasing prevalence, both in Brazil and other South American countries, it appears that the myopic shift and myopia progression are comparatively lower than in other regions worldwide(3,4). Furthermore, meta-analyses and population-based studies have revealed temporal trends showing an increased prevalence among children, particularly with a higher likelihood in girls compared to boys. The onset of myopia has been associated with parental myopia, and there has been a notable increase among adolescents, along with a higher prevalence in urban areas compared to rural areas in Asia(5-7).
The onset of myopia during the early school years (ages 5-7) is associated with faster progression and higher rates of myopia and eye growth throughout childhood and adolescence, especially among individuals exposed to environmental and behavioral factors related to myopia progression(1,5,8). The age of myopia onset and/or the duration of its progression are crucial predictors of high myopia in late childhood or adolescence(9). Children experiencing early onset and rapidly advancing progression are prone to developing high myopia (≤-6 diopters (D)), leading to potential complications in adulthood that may result in irreversible visual impairment(10,11).
Among the main complications associated with high myopia are myopic macular degeneration (affecting 1%-4% of the general population in some countries), cataract, glaucoma, retinal detachment, and optic neuropathy(12-13). Several environmental factors contribute to the changes in lifestyle that increase the risk of myopia progression. These factors include spending less time outdoors, high-pressure educational systems (especially in early ages in East Asian countries), continuous and excessive use of electronic devices (mainly tablets and cell phones) and engaging in other close-up activities(1,8). The objective of this study is to provide guidance on the evaluation and management of myopia progression in children.
METHODS
This guideline was developed by reviewing the existing literature and drawing upon the clinical expertise of experts from the Brazilian Society of Pediatric Ophthalmology (SBOP) and the Brazilian Society of Contact Lenses and Cornea (SOBLEC). The review encompassed the prevalence, classification, progression, and treatment of myopia, with data gathered from PubMed up to April 2023. The search utilized various terms such as myopia AND children OR classification OR prevalence OR progression OR treatment (progression AND myopia AND treatment). The group selected and analyzed 83 scientific papers, comprising meta-analyses, systematic reviews, randomized controlled trials (RCTs), case-control studies, observational studies, and case reports. To assess the quality of evidence, the studies were classified based on Guyatt et al.’s(14) criteria: Level I involved two or more high-quality RCTs, studies with high evidence level by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE), or statements from other guidelines with level A of evidence (experimental or observational studies with higher consistency). Level II evidence was established when there were a limited number of RCTs, multiple controlled but non-randomized studies, or several RCTs of lower quality. Additionally, evidence at this level could be derived from cohort or case-control studies, preferably conducted by multiple research groups or from multiple centers. Furthermore, clear-cut effects observed in non-controlled studies, studies with moderate evidence level according to GRADE, or statements from other guidelines with level B of evidence (experimental or observational studies with lower consistency) were also considered as Level II(14-15). On the other hand, Level III evidence relied on expert opinions, clinical experiences, descriptive studies(15-17), cohort or case-control studies of lower quality, studies with low or very low evidence level by GRADE, or statements from other guideline with level C or D of evidence (case reports studies or specialist opinion-based consensus)(14-17).
The final guideline document received approval from all the representatives of the involved societies. Since there was no involvement of human subjects, ethics approval was not required and was therefore waived.
RESULTS
The guidelines presented here for monitoring and treating myopia progression in children are based on a thorough review of the current literature and the clinical expertise of the expert group. This document encompasses the latest concepts on myopia classification, risk factors associated with myopia progression, recommended ophthalmologic visits and ancillary examination regimens, as well as strategies for preventing and treating myopia progression.
Myopia classification
Myopia is defined quantitatively as a condition where the spherical equivalent refractive error (SER), with relaxed ocular accommodation, is ≤-0.5 D. According to IMI, low myopia is characterized by a refractive error between ≤-0.5 and >-6.00 D, whereas high myopia is when the refractive error is ≤-6.00 D(17). However, it is important to note that the World Health Organization (WHO) classifies high myopia as a refractive error above -5.00 D(21,22).
Myopia can be further categorized into refractive and axial types. In refractive myopia, the optical power of the cornea and/or crystalline is high in eyes with a normal axial length (AL). Conversely, in axial myopia, the optical axis is too long compared to the refractive power of the cornea and lens. Axial and refractive myopia are often considered as distinct entities.
A definition for pre-myopia is also worth noting. It includes children with refractive error status of <+0.75 D at 6 years old, ≤+0.50 D between 7 and 8 years, ≤+0.25 D at 9-10 years, and ≤0 D at 11 years(16-18,21). Pre-myopia is of significance as it identifies children who are at a high risk of developing myopia in the future.
Risk factors for fast-progressing myopia
Based on current literature, myopia tends to progress faster in younger children and decelerates with age(1,22). Early onset of myopia or a prolonged duration of myopia progression are the most significant predictors of high myopia(2,5,23) (Level I). Heredity also plays a crucial role in myopia development, with approximately 150 genetic loci identified as contributing to the condition(24). Additionally, studies on parental myopia have revealed that having one myopic parent triples the risk of myopia, while having both parents with myopia increases the risk sevenfold(25,26). Various factors influence myopia progression, including ethnicity (more common among Asians), parents with a higher education level, less time spent outdoors, and engagement in schooling/near work activities(1).
A rapid myopia progression is considered to occur when there is an increase in refractive error increase rate of 0.75 D/year or higher(3,26). Risk factors for fast-progressing myopia include the following:
- Age younger than 7 years old(26) (Level I)
- Ethnicity (Asian)(26) (Level II)
- Parental myopia(26) (Level II)
- Limited time spent outdoors(27) (Level I)
- Prolonged durations of near work/screen time (>45 minutes), continuous use, and very close use (<25 cm)(28) (Level II)
Ophthalmologic evaluation
Myopia management requires adherence to a clinical protocol for closely monitoring the progression of the SER and AL. When myopic progression is suspected, an ophthalmologic evaluation should be conducted every 6 months, including medical history and the following components(18):
- Clinical history: Assessing visual impairment for distance, if applicable, age since commencement of wearing glasses, family history of myopia, and lifestyle risk factors (e.g., parents’ education level, outdoor time, near work-digital screen time, etc.)
- Examinations:
Best-corrected visual acuity for distance and near vision
Evaluation of accommodative and binocular vision
Anterior biomicroscopy
Cycloplegic refraction-utilizing the recommended cycloplegia protocol: one drop of 0.5% proxymetacaine, followed by one drop of 1% cyclopentolate, and then one drop of 1% tropicamide given 0-5 minutes apart. The test should be performed 30-40 minutes after the first drop(29).
Retinal imaging
Additional exams (if available):
- Annual measurement of AL (biannually in fast-progressing cases) using non-contact devices like Optical Biometry
- Corneal topography (to exclude keratoconus or to determine the requirement for contact lens fitting)
Myopic progression diagnosis and indication for treatment:
- Cycloplegic refractive error examination: an increase higher than SER -0.50 D over 1 year (with exceptions considered (≥0.5) for cases of early-onset myopia (<7 years) and with other fast-progressing risk factors)(30)
- AL: an axial growth of 0.3 mm/year (until 10 years old) or 0.2 mm/year (above 10 years old)
NOTE: Emmetropes typically have an AL of 22-24.5 mm, while axial lengths greater than 25 mm are generally associated with myopia(18).
Myopia prevention
Children with fast progression risk factors and those identified as pre-myopes should be carefully monitored to prevent or delay the early onset or progression of myopia. One RCT showed that increased time spent outdoors may offer some protection against myopia onset(30). Moreover, it can slow down myopia progression, although the effect might not be clinically significant. Therefore, encouraging outdoor activities should be viewed as an additional treatment to complement other interventions for myopia control (Level I), rather than a standalone solution(30,31). A population-based article conducted in Taiwan, which analyzed the myopic shift in preschool children (ages 5-6) encouraged to engage in outdoor activities (at least 30 minutes daily), reported a nearly 50% reduction in the incidence of myopia(32). It appears that increased outdoor exposure can reduce the risk of myopia onset, but it may not have a significant impact on myopic progression in individuals already diagnosed with the condition.
Treatment for reducing myopia progression
Atropine for myopic control
Atropine is a muscarinic drug, but its exact site of action remains undetermined. Different concentrations of atropine have been found to be effective in controlling myopia: low concentrations (0.01%, 0,025%, or 0.05%), medium concentrations (0.075%-0.1%), and high concentrations (above 0.1%)(33,34) (Level I). Control with low concentrations has been observed to be dose-dependent (Level I)(35), while higher concentrations have shown better myopia control (Level I)(36). However, the use of high concentrations can lead to more significant side effects, such as reduced amplitude of accommodation, glare, photophobia, and headaches(37).
Studies have indicated that low concentrations of atropine can control approximately 40%-70% of myopia progression in the Asian population(38-39) (Level I). The most effective long-term dose (balancing control, side effects, and rebound) has not yet been fully established. After discontinuing atropine treatment, some patients may experience a rebound effect (Level I)(36); therefore, the optimal duration of treatment remains uncertain. The current treatment involves administering one drop of atropine at night, to be continued for a minimum of 2 years, but it may extend until the child reaches 15-16 years old (Level I)(33,36-38). To avoid potential rebound effects, some researchers propose a gradual tapering of the atropine concentration instead of abrupt stop, although detailed studies on this approach are still lacking(30). The reduction can be accomplished by gradually reducing the concentration for at least 2 months, and once it reaches 0.01%, it can be used on alternate days for a brief period before discontinuation.
Using atropine at a low concentration in children aged 5 years and older is considered safe, whereas younger children may require a higher dose for effective myopia control (e.g., 0.05%)(34) (Level I). Adverse events related to atropine use are rare and may include allergies, mydriasis, and reduced accommodation. Therefore, discussing the use of atropine with the child’s family is essential. Atropine is not recommended for patients with astigmatism exceeding 1.50 or 2.50, corneal ectasia, neurological diseases, or those with a known allergy to atropine(37). Its use in individuals with Down Syndrome and high myopia lacks sufficient support from published studies; hence, predicting the expected outcomes or the safety of the atropine treatment in these cases is not possible(35,38).
According to this expert consensus, when treating with atropine for myopia control, it is recommended to follow a biannual follow-up schedule for monitoring refractive status (Level IIID) and annual follow-up for biometry (Level I). Based on the previous literature review (all Level I evidence) and clinical experience, several possibilities for atropine prescription for myopia control are presented below:
1. Begin with low-dose atropine (0.01%) for 1 year. If the desired control is not achieved, increase the dose to 0.025%. If still uncontrolled in the subsequent year, further increase the dose to 0.05%(30) (Level I).
2. Start the dose between 0.01% and 0.025% based on risk evolution criteria, such as child’s young age, parental history of myopia, myopia progression rate (MPR), refraction, and axial diameter.
3. For children aged between 5 and 8 years: Initiate treatment with 0.025% or 0.05% atropine. For children aged between 9 and 15 years:
a. If MPR is 0.50D/year or refraction is less than or equal to 4 D, and/or AXL is less than 24.5 mm, start with 0.01% atropine.
b. If MPR is higher than 0.50 D/year or refraction over -4 D, and/or AXL is larger than 24.5 mm, start with 0.025% atropine.
For patients aged over 15 years: Initiate treatment with 0.01% atropine (Level I)(33,35,36,39).
Hyperopic defocus for myopia treatment
Peripheral hyperopic defocus (PHD) refers to an optical abnormality where hypermetropia (farsightedness) induced in the midperiphery of the retina causes a phenomenon of defocusing. This occurs due to the conical shape of the eye, leading to hypermetropic blur in the peripheral retina. The peripheral retina is believed to play a role in controlling AL growth, and the presence of PHD has been recognized as a potential risk factor for myopia progression in humans(40-42). Hung et al.(43) (Level III) proposed that an increase in the area of PHD can result in a reduction of neuromodulators, such as dopamine, being released in the peripheral retina. This increase in the area of PHD is linked to the weakening of the structural integrity of the sclera, which consequently leads to an increase in AL.
Lens wear in the hypermetropic defocus treatment
Based on the peripheral defocus theory, new custom-designed lenses aim to minimize PHD. Several recent ophthalmic lens designs for myopia control are as follows:
1) Defocus Incorporated Multiple Segments (DIMS)/Multisegment of Myopic Defocus Spectacle Lens (developed by Hoya)(44). In a randomized study, it was found that myopia progression was significantly reduced by 59% and AL elongation decreased by 60% when compared to wearing single-vision lenses (Level I)(45). Another 2-year randomized clinical trial involved children aged 8-13 years (n=160) with myopia from -1.00 to -5.00 D and astigmatism up to 1.50 D. The trial compared children using single-vision glasses with those using DIMS lenses. The results showed that children wearing DIMS lenses had 52% less myopia progression and 62% less axial elongation compared to the control group. Additionally, 21.5% of DIMS lens wearers experienced no myopia progression during the study period, in contrast to 7% of those wearing single-vision glasses (Level I)(44). After a 3-year period, 120 children who initially wore single-vision glasses switched to using DIMS lenses. The positive results in myopia control were sustained over an additional 3 years (n=65). Even those children who underwent DIMS lens replacement (n=55) responded well to the treatment, as evident from an improved progression curve (Level I)(45). In a recent study with a 6-year follow-up, the paper evaluated four groups: Group 1 (n=36) wore DIMS spectacles for 6 years; Group 2 (n=14) wore DIMS lens for the first 3.5 years and switched to SV spectacles afterward; Group 3 (n=22) used SV spectacles for the first 2 years and then switched to DIMS lenses; Group 4 (n=18) wore SV spectacles for the first 2 years, then switched to DIMS lenses for 1.5 years, and finally returned to SV spectacles again. The study findings revealed that Group 1 showed no significant differences in myopia progression (-0.52 ± 0.66 vs. -0.40 ± 0.72 D) and axial elongation (0.32 ± 0.26 vs. 0.28 ± 0.28 mm, both p>0.05) between the first and subsequent 3 years. In the last 2.5 years, the groups using DIMS lenses (Groups 1 and 3) displayed less myopia progression and axial elongation than the groups using single-vision lenses (Groups 2 and 4)(46) (Level I).
2) Highly Aspherical Lenslet Target or HALT Technology (developed by Essilor)(47). This technology demonstrated a remarkable 67% deceleration in myopia progression, on average, compared to single-vision lenses worn for 12 hours. A two-year randomized study with 157 children aged 8-13 years showed a significant reduction in myopia progression for both SE and AL by 67% (0.99 D) and 60% (0.41 mm), respectively, when compared to single-vision lenses (Level I)(48).
Lenses equipped with DIMS and HALT technology share the following characteristics:
• A single central vision zone for distance
• A single-vision correction in the peripheral zone
• An intermediate zone (treatment area) containing multiple segments (DIMS) or lenslets (HALT) designed to create a differential myopic blur in front of the retina, with spaces between them for single-vision correction
• No alteration in binocular vision or accommodation
• A strict requirement for proper centralization of the lens on the central clear zone to ensure better acuity and defocus treatment in the midperiphery, as well as no serious vision complaints for distance and middle periphery
3) Perifocal defocus spectacle lens (developed by IOT/Art Optica). This lens creates a positive asymmetric defocus (largest in the temporal area) on the horizontal meridian. In a study involving a Caucasian population, after 5 years, myopia progression was significantly reduced from -1.95 ± 0.2 D in the control group versus -1.16 ± 0.2 D in the treated group, with a difference of 0.79 D between them. The AL elongation was decreased by 56% (0.46 mm ± 0.05 vs 0.71 ± 0.09 mm) in 2 years when compared to wearing single-vision lenses(49). (Level I).
Defocus contact lenses in myopia control
Myopia control studies with multifocal contact lenses have yielded varying results, with reductions in myopia progression rates (SE) ranging from 0 to 72% to 80%(50-52).
One notable study called BLINK(53) (Bifocal Lenses in Nearsighted Kids) compared multifocal CL with simple vision CL in 292 children aged between 7 and 11 years. The study, which had a follow-up period of 3 years, demonstrated a reduction in myopia progression by 43% and AL by 36% (Level I). The efficacy of the treatment was more significant in the higher addition group (+2.50 D addition), which could be particularly relevant for younger children who have positive individual risk factors for myopia progression.
The CooperVision MiSight lens is currently the only disposable lens approved in Brazil for myopia control. A 7-year clinical study involving the MiSight lens showed no apparent rebound effect (Level I)(54). In a multicenter, prospective, randomized, double-blind study, MiSight lenses were compared with single-vision spherical disposable soft lenses in children aged 8-12 years with myopia ranging from -0.75 to -4.00 D and astigmatism <1.00 D. The study revealed reductions in myopia progression (59%) and AL (52%) during a 3-year follow-up period (Level I)(54). After 6 years of follow-up, 23% of the patients experienced a refractive change of less than 0.25 D (spherical equivalent). Even the original control group, which switched to MiSight lenses in the fourth year of follow-up also, exhibited a reduction in myopia progression and AL growth (0.81 mm) over the subsequent 3 years.
ORTHO-K in myopia control
Orthokeratology is a technique used for temporary reduction of refractive errors. It involves using specially designed reverse curve contact lenses that apply positive pressure to the center of the cornea, aiming to reshape it. The lens effects are reversible and can either disappear within a few hours or last for a day or longer. This treatment has no age restrictions and can be used for individuals of all ages. It works by reducing the thickness of the central epithelium of the cornea through the redistribution of intracellular fluid from these cells to the intracellular space of the midperipheral epithelial cells. The contact lens exerts negative pressure at the midperipheral region, leading to the thickening of the corneal epithelium in that area. It is important to note that no cellular displacement occurs during this process; instead, there is a redistribution of intracellular fluid within the corneal epithelium(55).
Corneal remodeling is responsible for a temporary reduction in the AL of the eye, effectively correcting myopia. According to findings in published literature, this corneal remodeling also contributes to reducing myopia progression in 35%-60% of patients. It achieves by forming an elevation in the corneal midperiphery, which results from thickening induced by the migration of intracellular fluid. This elevation leads to a refractive alteration that corrects the hypermetropic defocus in the midperiphery of the retina (Level I and II)(54-61). For routine evaluation, in addition to the standard examinations used for myopic children evaluation, computerized topography with axial and tangential maps, as well as specular corneal microscopy and tomography (Galilei or Pentacam), are recommended as complementary examinations.
Patients with regular corneas are generally more suitable for orthokeratology treatment. Existing literature indicates that the adverse effects of orthokeratology treatment are reversible and comparable to those seen with other types of contact lenses designed for night wear(62-68). However, there are some contraindications to consider:
a. Clinical contraindications: Patients with inflammation or infections in the anterior segment of the eye (bacterial, viral, or fungal); abnormalities in the conjunctiva, cornea, or eyelid; corneal hypoesthesia; dry eye; systemic conditions that affect the eyes, the lacrimal pathways, and tearing should not undergo orthokeratology treatment.
b. Corneal contraindications: Patients with against-the-rule astigmatism, corneal ectasia, astigmatism exceeding half the spherical degree, cylinders larger than 1.75 D, and corneas flatter than 40 D or more curved than 48 D and those who, after treatment, show a corneal curvature below 38 D should not be considered for orthokeratology treatment.
Combined treatments
Few studies have explored the association of treatments, although it is possible that a combined effect exists when treatments with different mechanisms of action are used together. Some studies have suggested that combining atropine and Ortho-K treatment may lead to a greater effect in slowing axial elongation in children with myopia compared to Ortho-K monotherapy (Level II)(69). A recent meta-analysis comparing Ortho-K to Ortho-K plus atropine found four eligible studies with a total of 267 subjects (Level I)(70). The analysis revealed that the mean AL in the experimental group was 0.09 mm shorter than in the control group (WMD=-0.09, 95%CI [-0.15, -0.03], p=0.003). However, no significant differences were observed between the two groups in terms of uncorrected distant visual acuity, corneal endothelial cell density, or intraocular pressure (WMD was -0.01 [95% CI: -0.03, 0.01], 11.75 [95% CI: -4.09, 27.58], 0.12 [95% CI: -0.40, 0.63], respectively). None of the studies reported severe adverse events. Kinoshita et al. reported a better combined treatment effect, especially in the first year(69)(0.09 mm vs. 0.19 mm), but the difference between the two treatments diminished in the second year (0.20 mm vs. 0.21 mm).
The combination treatment showed a more pronounced additive effect in slowing axial growth among children with lower myopia, whereas monotherapy was equally effective as the combination in children with higher myopia. The key factor lies in the myopia correction achieved with Ortho-K therapy. In children with higher myopia, Ortho-K provides a larger myopia correction, resulting in improved defocus on the peripheral retina. Conversely, in children with lower myopia, the extent of myopia correction with Ortho-K is smaller, and this might not sufficiently improve the defocus on the peripheral retina through monotherapy alone. In such cases, adding 0.01% atropine seems to be more effective. The study suggests that combining Ortho-K and 0.01% atropine yields greater effectiveness in slowing axial elongation in children with myopia, particularly in cases with a relatively short duration of treatment.
In a recent study, conducted in a European population with 146 participants, the participants were divided into 4 groups: 53 received 0.01% atropine, 30 used DIMS spectacles, 31 received a combination of 0.01% atropine and DIMS, and 32 used the single-vision control spectacles. After 1 year of treatment, the group receiving the atropine and DIMS showed a significant reduction in myopia progression compared to both the DIMS-only and atropine-only groups. Interestingly, during this period, there was no difference in myopia progression between the atropine and DIMS groups(71) (Level II).
DISCUSSION
The strategies described here play a crucial role in managing myopia progression and are essential to minimize the number of high myopes and their possible consequences. The choice of treatment should be informed by the expertise of the healthcare professional and the socioeconomic context. It is important to acknowledge that in countries like Brazil, with limited resources and as a low-income country, access to therapies such as glasses or contact lenses may not be available for most of the population. Thus, this factor needs to be carefully considered before making the initial prescription.
Suggested treatment strategies (Flowchart)
Based on the current scientific evidence and the consensus of the professionals involved in developing the guidelines and the Brazilian reality, a flowchart has been created for the follow-up and control of myopic children (Figure 1).
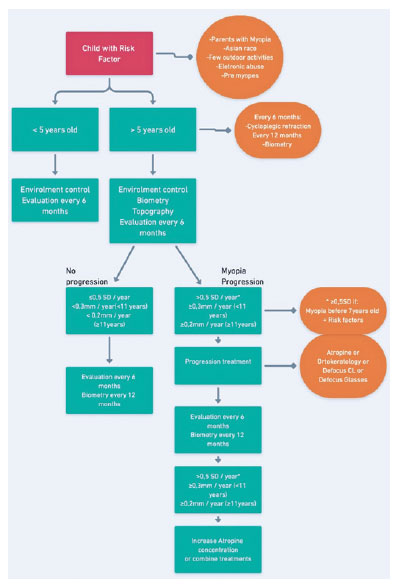
When choosing the appropriate method, healthcare professionals should take into account various factors such as cost, astigmatism (defocus CL correcting up to 0.75 DC), ophthalmologist’s personal experience, and the perception of invasiveness associated with a particular treatment.
For cases where monotherapy proves insufficient in controlling myopia progression, treatment options may involve increasing atropine concentration (in cases where the drug is already being used) or combining it with another therapeutic approach. In such situations, the decision should consider the healthcare professional’s personal experience or familiarity with the selected method.
The treatment duration is determined based on the refractive error stability and the AL (Figure 1).
REFERENCES
1. Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, Rose KA. The epidemics of myopia: etiology and prevention. Prog Retin Eye Res. 2018 Jan;62:134-49.
2. Morgan IG, Wu PC, Ostrin LA, Tideman JW, Yam JC, Lan W, et al. IMI Risk Factors for Myopia. Invest Ophthalmol Vis Sci. 2021;62(5):3.
3. Gaiotto PC, Passos Junior W, Schellini SA, Shiratori CA, Padovani CR. Afecções oculares em crianças de 2 a 8 anos da rede pública municipal de Piracicaba-SP. Medicina (Ribeirão Preto). 2002;35(4):487-91.
4. Garcia CA, Orefice F, Nobre GF, Souza DB, Rocha ML, Vianna RN. [Prevalence of refractive errors in students in Northeastern Brazil]. Arq Bras Oftalmol. 2005;68(3):321-5. Portuguese.
5. Dong L, Kang YK, Li Y, Wei WB, Jonas JB. Prevalence and time trends of myopia in children and adolescents in china: A systemic review and meta-analysis. Retina. 2020;40(3):399-411.
6. Jones LA, Sinnott LT, Mutti DO, Mitchell GL, Moeschberger ML, Zadnik K. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48(8): 3524-32.
7. Li SM, Wei S, Atchison DA, Kang MT, Liu L, Li H, et al. Annual incidences and progressions of myopia and high myopia in Chinese schoolchildren based on a 5-year cohort study. Invest Ophthalmol Vis Sci. 2022;63(1):8. 8. Rose KA, Morgan IG, Smith W, Burlutsky G, Mitchell P, Saw S-M. Myopia, lifestyle, and schooling in students of Chinese ethnicity in Singapore and Sydney. Arch Ophthalmol. 2008;126(4):527-30.
9. Congdon N, Burnett A, Frick K. The impact of uncorrected myopia on individuals and society. Commun Eye Health. 2019;32(105):7-8.
10. Chua SY, Sabanayagam C, Cheung YB, Chia A, Valenzuela RK, Tan D, et al. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016;36(4):388-94.
11. Tideman JW, Snabel MC, Tedja MS, van Rijn GA, Wong KT, Kuijpers RW, et al. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol. 2016; 134(12):1355-63.
12. Haarman AE, Enthoven CA, Tideman JW, Tedja MS, Verhoeven VJ, Klaver CC. The complications of myopia: a review and meta-analysis. Invest Ophthalmol Vis Sci. 2020;61(4):49.
13. Wu X, Gao G, Jin J, Hua W, Tao L, Xu S, et al. Housing type and myopia: the mediating role of parental myopia. BMC Ophthalmol. 2016;16(1):151.
14. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-6.
15. Prousali E, Haidich AB, Fontalis A, Ziakas N, Brazitikos P, Mataftsi A. Efficacy and safety of interventions to control myopia progression in children: an overview of systematic reviews and meta-analyses. BMC Ophthalmol. 2019;19(1):106.
16. Walline JJ, Lindsley KB, Vedula SS, Cotter SA, Mutti DO, Ng SM, et al. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020 Jan 13;1(1):CD004916.
17. IMI Clinical Management Myopia Guidelines Report. [cited 202 June 30]. Available from: https://myopiainstitute.org/imi-whitepaper/clinical-management-myopia-guidelines-report/
18. Jong M, Jonas JB, Wolffsohn JS, Berntsen DA, Cho P, Clarkson-Townsend D, et al. IMI 2021 Yearly Digest. Invest Ophthalmol Vis Sci. 2021;62(5):7.
19. World Health Organization (WHO). The impact of myopia and high myopia: report of the joint World Health Organization-Brien Holden Vision Institute Global Scientific Meeting on Myopia, University of New South Wales, Sydney, Australia, 16-18 March 2015. Genova: WHO; 2017.
20. World Health Organization (WHO). World report on vision. Genova: WHO; 2019.
21. Flitcroft DI, He M, Jonas JB, Jong M, Naidoo K, Ohno-Matsui K, et al. IMI - defining and classifying myopia: a proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019;60(3):M20-30.
22. Jonas JB, Ang M, Cho P, Guggenheim JA, He MG, Jong M, et al. IMI prevention of myopia and its progression. Invest Ophthalmol Vis Sci. 2021;62(5):6.
23. Han X, Liu C, Chen Y, He M. Myopia prediction: a systematic review. Eye (Lond). 2022;36(5):921-9.
24. Wu LJ, You QS, Duan JL, Luo YX, Liu LJ, Li X, et al. Prevalence and associated factors of myopia in high-school students in Beijing. PLoS One. 2015;10(3):e0120764.
25. O’Donoghue L, Kapetanankis VV, McClelland JF, Logan NS, Owen CG, Saunders KJ, et al. Risk factors for childhood myopia: findings from the NICER study. Invest Ophthalmol Vis Sci. 2015; 56(3):1524-30.
26. Foreman J, Salim AT, Praveen A, Fonseka D, Ting DS, He M, et al. Association between digital smart device use and myopia: a systematic review and meta-analysis. Lancet Digit Health. 2021;3(12):e806-18.
27. Wu PC, Chen CT, Lin KK, Sun CC, Kuo CN, Huang HM, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-50.
28. Li SM, Li SY, Kang MT, Zhou Y, Liu LR, Li H, et al. Near work related parameters and myopia in Chinese children: the Anyang Childhood Eye Study. PLoS One. 2015;10(8):e0134514.
29. Curi I, Nakayama SA , Pereira EM, Hopker LM, Ejzenbaum F, Barcellos RB, et al. Brazilian guideline for pediatric cycloplegia and mydriasis. Arq Bras Oftalmol. 2023;86(4):388-96.
30. Wu PC, Chuang MN, Choi J, Chen H, Wu G, Ohno-Matsui K, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond.) 2019;33(1):3-13.
31. Xiong S, Sankaridurg P, Naduvilath T, Zang J, Zou H, Zhu J, et al. Time spent in outdoor activities in relation to myopia prevention and control: a meta-analysis and systematic review. Acta Ophthalmol. 2017; 95(6):551-66.
32. Yang YC, Hsu NW, Wang CY, Shyong MP, Tsai DC. Prevalence trend of myopia after promoting eye care in preschoolers: a serial survey in Taiwan before and during the coronavirus disease 2019 pandemic. Ophthalmology. 2022;129(2):181-190
33. Huang J, Wen D, Wang Q, McAlinden C, Flitcroft I, Chen H, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4): 697-708.
34. Gan J, Li SM, Wu S, Cao K, Ma D, He X, et al. Varying dose of atropine in slowing myopia progression in children over different follow-up periods by meta-analysis. Front Med (Lausanne). 2022 Jan 13;8:756398.
35. Yam JC, Jiang Y, Tang SM, Law AK, Chan JJ, Wong E, et al. Low-concentration atropine for myopia progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology. 2019;126(1):113-24.
36. Ha A, Kim SJ, Shim SR, Kim YK, Jung JH. Efficacy and safety of 8 atropine concentrations for myopia control in children: a network meta-analysis. Ophthalmology. 2022;129(3):322-33.
37. Chia A, Chua WH, Cheung YB, Wong WL, LIngham A, Fong A, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (ATOM2). Ophthalmology. 2012;119(2):347-54.
38. Cooper J, Eisenberg N, Schulman E, Wang FM. Maximum atropine dose without clinical signs or symptoms. Optom Vis Sci. 2013; 90(12):1467-72.
39. Fang PC, Chung MY, Yu HJ, Wu PC. Prevention of myopia onset with 0.025% atropine in premyopic children. J Ocul Pharmacol Ther. 2010;26(4):341-5.
40. Li FF, Zhang Y, Zhang X, Yip BH, Tang SM, Kam KW, et al. Age effect on treatment responses to 0.05%, 0.025%, and 0.01% atropine: low-concentration atropine for myopia progression study. Ophthalmology. 2021;128(8):1180-7.
41. Smith EL, Kee CS, Ramamirtham R, Qiao-Grider Y, Hung LF. Peripheral vision can influence eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2005;46(11):3965-72.
42. Smith EL 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS, et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48(9):3914-22.
43. Smith EL 3rd, Huang J, Hung LF, Blasdel TL, Humbird TL, Bockhorst KH. Hemiretinal form deprivation: evidence for local control of eye growth and refractive development in infant monkeys. Invest Ophthalmol Vis Sci. 2009;50(11):5057-69.
44. Hung GK, Ciuffreda KJ. A unifying theory of refractive error development. Bull Math Biol. 2000;62(6):1087-108.
45. Lam CS, Tang WC, Tse DY, Lee RP, Chun RK, Hasegawa K, et al. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: a 2-year randomized clinical trial. Br J Ophthalmol. 2020;104(3):363-8.
46. Lam CS, Tang WC, Zhang HY, Lee PH, Tse DY, Qi H, et al. Long- -term myopia control effect and safety in children wearing DIMS spectacle lenses for 6 years. Sci Rep. 2023;13(1):5475.
47. Lam CS, Tang WC, Lee PH, Zhang HY, Qi H, Hasegawa K, et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br J Ophthalmol. 2022; 106(8):1110-4.
48. Bao J, Yang A, Huang Y, Li X, Pan Y, Ding C, et al. One-year myopia control efficacy of spectacle lenses with aspherical lenslets. Br J Ophthalmol. 2022; 106(8):1171-6.
49. Tarutta EP, Proskurina OV, Tarasova NA, Milash SV, Markosyan GA. [Long-term results of perifocal defocus spectacle lens correction in children with progressive myopia]. Vestn Oftalmol 2019;135(5):46-53. Russian.
50. Bao J, Huang Y, Li X, Yang A, Lim EW, Zheng J, et al. Myopia control with spectacle lenses with aspherical lenslets: a 2-year randomized clinical trial. Invest. Ophthalmol Vis Sci. 2021;62(8):2888.
51. Sankaridurg P, Bakaraju RC, Naduvilath T, Chen X, Weng R, Tilia D, et al. Myopia control with novel central and peripheral plus contact lenses and extended depth of focus contact lenses: 2-year results from a randomised clinical trial. Ophthalmic Physiol Opt. 2019;39(4):294-307.
52. Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, Prieto-Garrido FL, Gutiérrez-Ortega R, Villa-Collar C. MiSight Assessment Study Spain (MASS): a 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1011-21.
53. Walline JJ, Walker MK, Mutti DO, Jones-Jordan LA, Sinnott LT, Giannoni AG, et al. BLINK Study Group. Effect of high add power, medium add power, or single-vision contact lenses on myopia progression in children: the BLINK randomized clinical trial. JAMA. 2020;324(6):571-80.
54. Chamberlain P, Bradley A, Arumugam B, Hammond D, McNally J, Logan NS, et al. Long-term effect of dual-focus contact lenses on myopia progression in children: A 6-year Multicenter Clinical Trial. Optom Vis Sci. 2022;99(3):204-12.
55. Choo JD, Caroline PJ, Harlin DD, Papas EB, Holden BA. Morphologic changes in cat epithelium following continuous wear of orthokeratology lenses: A pilot study. Cont Lens Anterior Eye. 2008;31(1):29-37.
56. Cho P, Cheung SW. Retardation of myopia in Orthokeratology (ROMIO) study: a 2-year randomized clinical trial. Invest Ophthalmol Vis Sci. 2012;53(11):7077-85.
57. Hiraoka T, Kakita T, Okamoto F, Oshika T. Influence of ocular wavefront aberrations on axial length elongation in myopic children treated with overnight orthokeratology. Ophthalmology. 2015; 122(1):93-100.
58. Santodomingo-Rubido J, Villa-Collar C, Gilmartin B, Gutiérrez-Ortega R. Myopia control with orthokeratology contact lenses in Spain: refractive and biometric changes. Invest Ophthalmol Vis Sci. 2012;53(8):5060-5.
59. Chen Z, Niu L, Xue F, Qu X, Zhou Z, Zhou X, et al. Impact of pupil diameter on axial growth in orthokeratology. Optom Vis Sci. 2012;89(11):1636-40.
60. Chen C, Cheung SW, Cho P. Myopia control using toric orthokeratology (TO-SEE Study). Invest Ophthalmol Vis Sci. 2013;54(10):6510-7.
61. Lipson MJ, Brooks MM, Koffler BH. The role of orthokeratology in myopia control: a review. Eye Contact Lens 2018;44(4):224-30.
62. Young K. Slowing myopia: CooperVision’s Paragon CRT contact lenses receive CE mark. Optometry Today; 2020 [cited 202e June 30]. Available from: https://www.aop.org.uk/ot/industry/contact-lenses/2020/10/07/slowing-myopia-coopervisions-paragon-crt-contact-lenses-receive-ce-mark
63. Efron N. Contact lens complications. Butterworth Heineman: Elsevier; 2004. p. 69-75.
64. Liu YM, Xie P. The safety of orthokeratology - a systematic review. Eye Contact Lens 2016;42(1):35-42.
65. Cheng HC, Liang JB, Lin WP, Wu R. Effectiveness and safety of overnight orthokeratology with Boston XO2 high-permeability lens material: A 24 week follow-up study. Cont Lens Anterior Eye. 2016;39(1):67-71.
66. Bullimore MA, Sinnott LT, Jones-Jordan LA. The risk of microbial keratitis with overnight corneal reshaping lenses. Optom Vis Sci. 2013;90(9):937-44.
67. Walline JJ, Rah MJ, Jones LA. The Children’s Overnight Orthokeratology Investigation (COOKI) pilot study. Optom Vis Sci. 2004;81(6):407-13.
68. Verzhanskaya TY, Tarutta EP. [Stabilizing effectiveness of orthokeratology and long-term minute-concentration atropine therapy in myopia (draft report)]. Vestn Oftalmol. 2017;133(5):43-8. Russian.
69. Kinoshita N, Konno Y, Hamada N, Kanda Y, Shimmura-Tomita M, Kakehashi A. Additive effects of orthokeratology and atropine 0.01% ophthalmic solution in slowing axial elongation in children with myopia: first year results. Jpn J Ophthalmol. 2018;62(5):544-53.
70. Wang S, Wang J, Wang N. Combined orthokeratology with atropine for children with myopia: a meta-analysis. Ophthalmic Res. 2021;64(5):723-31.
71. Nucci P, Lembo A, Schiavetti I, Shah R, Edgar DF, Evans BJ. A comparison of myopia control in European children and adolescents with defocus incorporated multiple segments (DIMS) spectacles, atropine, and combined DIMS/atropine. PLoS One. 2023;18(2):e0281816.
Submitted for publication:
January 16, 2023.
Accepted for publication:
June 14, 2023.
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.