

Magda Massae Hata Viveiros1; Luís Henrique Zucoloto2; Álvio Issao Shiguematsu1; Cláudia Aparecida Rainho3; Silvana Artioli Schellini1
DOI: 10.5935/0004-2749.2022-0084
ABSTRACT
PURPOSES: To determine the best protocol in obtaining the higher yield of conditioned culture medium to be used for the bone marrow mesenchymal stem cell differentiation into corneal epithelial cells, five techniques for the primary culture of human corneal epithelial cells were evaluated.
METHODS: The studied culture techniques of corneal epithelial cells were: explants in culture flasks with and without hydrophilic surface treatment, on amniotic membrane, with enzymatic digestion, and by corneal scraping. The conditioned culture medium collected from these cultures was used to differentiate human bone marrow mesenchymal stem cells into corneal epithelial cells, which were characterized using flow cytometry with pan-cytokeratin and the corneal-specific markers, cytokeratin 3 and cytokeratin 12.
RESULTS: The culture technique using flasks with hydrophilic surface treatment resulted in the highest yield of conditioned culture medium. Flasks without surface treatment resulted to a very low success rate. Enzymatic digestion and corneal scraping showed contamination with corneal fibroblasts. The culture on amniotic membranes only allowed the collection of culture medium during the 1st cell confluence. The effectiveness of cell differentiation was confirmed by cytometry analysis using the collected conditioned culture medium, as demonstrated by the expressions of cytokeratin 3 (95.3%), cytokeratin 12 (93.4%), and pan-cytokeratin (95.3%).
CONCLUSION: The culture of corneal epithelial cell explants in flasks with hydrophilic surface treatment is the best technique for collecting a higher yield of conditioned culture medium to be used to differentiate mesenchymal stem cells.
Keywords: Cell culture; Mesenchymal stem cells; Cell differentiation; Epithelial cells; Cornea; Conditioned culture medium; Culture techniques
RESUMO
OBJETIVOS: Foram estudadas cinco técnicas de cultivo primário de células epiteliais de córnea humana para se determinar o melhor protocolo para a obtenção do maior rendimento de meio de cultivo condicionado para ser utilizado na diferenciação de células tronco mesenquimais para células epiteliais de córnea.
MÉTODOS: As técnicas de cultivo estudadas foram: explantes em frascos de cultivo com e sem tratamento hidrofílico de superfície, sobre membrana amniótica, com digestão enzimática e por raspado de córnea. O meio de cultivo condicionado foi coletado e as células tronco mesenquimais induzidas a se diferenciarem em células epiteliais da córnea utilizando o meio de cultivo condicionado. As células foram caracterizadas por citometria de fluxo com pan-citoqueratina e com os marcadores específicos da córnea, citoqueratina 3 e citoqueratina 12.
RESULTADOS: A técnica utilizando frascos com o tratamento de superfície apresentou o maior rendimento de meio de cultivo condicionado. Os frascos sem tratamento de superfície levaram a uma taxa de sucesso muito baixa. A digestão enzimática e a raspagem da córnea mostraram contaminação das culturas com fibroblastos de córnea. A cultura sobre membranas amnióticas só permitiu a coleta do meio de cultivo condicionado durante a 1ª confluência celular. A análise de citometria de fluxo confirmou o sucesso da diferenciação celular utilizando o meio de cultivo condicionado coletado, demonstrada pela expressão de citoqueratina 3 (95,3%), citoqueratina 12 (93,4%) e pan-citoqueratina (95,3%).
CONCLUSÃO: O cultivo de explantes de células tronco mesenquimais em frascos com tratamento hidrofílico de superfície é a melhor técnica para a obtenção de um alto rendimento de meio de cultivo condicionado.
Descritores: Cultivo de células; Células tronco mesenquimais; Diferenciação celular; Células epiteliais; Córnea; Meio de cultivo condicionado; Técnicas de cultivo
INTRODUCTION
To recover transparency of injured cornea(1), advances in regenerative medicine are focused on corneal cell transference. Mesenchymal stem cells (MSCs) are currently the subject of great scientific interest, particularly in the field of ocular surface regeneration. MSCs are a type of multipotent progenitor cells(2), which have the ability to auto-regenerate as undifferentiated cells, and with the possibility to differentiate into lineages of mesenchymal tissues such as bone, cartilage, fat, muscle(3,4), cardiac muscle(5), neurons(6), and corneal epithelial cells (CEC)(7-11). Autologous MSCs can be collected, expanded, and differentiated using in vitro techniques and were successfully applied.
Cellular differentiation can be induced by conditioned culture medium (CM). When conditioning cells are cultivated, they secrete mediator substances into the cultivated medium, which can be preserved after the original conditioning cells are removed, generating the CM. This CM contains conditioning cell mediator substances, and when used on MSCs, these cells can develop the characteristics of the conditioning cells(12). To induce differentiation, it is essential to use a cell culture technique that allows rapid cell proliferation and a high yield of CM from the conditioning cell cultures.
This study aimed to evaluate five techniques of primary CEC culture, to determine the protocol with the highest yield of CM from these cultures to be used in MSC differentiation into CEC.
METHODS
Corneal epithelial cell primary culture techniques
The study protocol used was approved by the Institutional Research Ethics Committee (ERB #1.376.415). The study was conducted according to the tenets of the Declaration of Helsinki. A signed informed consent was obtained from the first-degree families of the corneal donors authorizing their use for treatment or scientific purposes. Moreover, a signed informed consent was obtained from the membrane donor authorizing its use for research purposes.
Ten human corneas discarded for use in corneal transplants due to low endothelial cell counts were used to culture the CEC. The limbal tissues were cultured separately from the corneal buttons, and the Descemet’s membrane, including the endothelial layer, was peeled off. The mean age of the corneal donors was 55.25 years (minimum, 29 years; maximum, 67 years).
Under sterile conditions, in a laminar flow hood, the corneal buttons and limbal tissues were washed with Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) (Gibco, Grand Island, NY, USA) with 100 IU/mL penicillin, 40 µg/mL gentamicin, and 2 µg/mL amphotericin-B (Gibco, Grand Island, NY, USA), and were cultured using the following five techniques:
- Culture of explants in flasks without hydrophilic surface treatment: The samples were cut into small fragments of approximately 2 mm3, which were seeded in 25 cm2 culture flasks without hydrophilic surface treatment, for a total of 100 cultures. Each explant was maintained with a drop of bovine fetal serum (BFS) for 30 min, allowing for adherence of the explants to the culture plate, before carefully adding the nutrient medium only to cover the explants, without allowing them to float.
- Culture of explants in flasks with hydrophilic surface treatment: The samples were cut into 2 mm3 explants, incubated for 30 min with a drop of BFS over each fragment allowing for explant attachment in tissue culture flasks with hydrophilic surface treatment (CellBIND culture flask, Corning, New York, NY, USA). The nutrient medium was added only until the explants were covered, without floating.
- Culture of explants on amniotic membrane (AM): The AM was obtained at the time of a cesarean section from a patient with negative serological tests for HIV-1, hepatitis B, hepatitis C, and syphilis. Under sterile conditions, the AM was washed three times using 0.9% physiological solution and once using phosphate buffer solution (PBS) containing 1,000 U/mL of penicillin, 20 mg/mL of streptomycin, and 2.5 mg/mL of amphotericin-B (Gibco, Grand Island, NY, USA). The amnion was separated from the chorion, and the AM was layered over sterile nitrocellulose filter papers of 3 × 3 mm, with the epithelial leaflet facing up. The AM fragments were stored at -80°C in DMEM-F12 nutrient medium with glycerol at a 1:1 dilution. Immediately before use, the AM was thawed for 30 min at room temperature, washed three times using PBS, and incubated for 15 min using 1 ml trypsin-EDTA (Gibco, Grand Island, NY, USA). The amniotic epithelial cells were removed by gentle scraping using cell scrapers. Once de-epithelialization was complete, the AM was washed twice using PBS to remove the cell debris and was attached to the bottoms of 35 mm Petri dishes with the basement membrane facing up; this enabled it to act as a substrate for the adherence of explants, which were cut into 2 mm3 fragments and placed on the surface.
- Culture with enzymatic digestion: The corneal tissues were cut into 2 mm3 fragments and were submitted to enzymatic digestion using 0.2% collagenase 2 for 30 min, centrifuged for 10 min at 1,200 rpm, resuspended in 2 ml nutrient medium, and placed in 24-well culture plates.
- Corneal scraping: The corneas were scraped with surgical blades to remove the epithelial cells, which were seeded in 25 cm2 tissue culture flasks with nutrient medium.
The nutrient medium for the CEC cultures used in all five techniques was Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) (Gibco, Grand Island, NY, USA) supplemented with 5 ml/L of TC Minimal Eagle vitamins (Sigma-Aldrich, St. Louis, MO, USA), 0.01 U/mL recombinant human insulin (Gibco, Grand Island, NY, USA), 15 µg/mL glutathione (Sigma-Aldrich, St. Louis, MO, USA), 100 IU/mL penicillin, 40 µg/mL gentamicin, 2 µg/mL amphotericin-B (Gibco, Grand Island, NY, USA), and 20% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA). The cultures were incubated at 37ºC, with 5% CO2, in a humid atmosphere. The nutrient medium was changed every 3 days until reach semi-confluence, occupying 60%-70% of the culture flask, when the CM was collected in each medium exchange until the third passage, filtered with a 0.2 µm micro-filter (Axygen, Glendale, AZ, USA), and stored at 4°C-8°C until use.
Mesenchymal stem cell primary cultures
The human bone marrow (BM) sample was obtained from a 46-year-old patient, during a surgical procedure for orthopedic humeral osteosynthesis. MSCs were harvested according to Ramakrishnan et al.(13). These cells were characterized to ensure they possessed the phenotypic and functional criteria used to identify MSCs, which include their ability to adhere to plastic surfaces, the expression of specific cell surface antigens, and their potential to differentiate into lineages of mesenchymal tissues, including osteocytes and adipocytes(4,14).
Third-passage MSCs were phenotypically characterized using flow cytometry using the following panel of markers: CD73, CD90, CD105, CD34, CD45, CD11b, CD19, and HLA-DR (BD Stemflow hMSC Analysis Kit, Becton Dickinson Pharmingen, Sparks, MD, USA). Briefly, cells were detached by trypsinization, and 100 µL of the cell suspension at a concentration of 5-10 × 106 cells/mL were dispensed into microtubes and were mixed with monoclonal antibodies or their respective isotypic controls (Table 1) and were incubated for 30 min at 2oC-8oC, protected from light. Cells were washed twice using cold PBS containing 10% FBS, were resuspended in 250 µl of the same solution, and were placed in cytometry tubes. Furthermore, data were immediately acquired using a FACS Canto II (BD Biosciences, San Jose, CA, EUA) flow cytometer and were analyzed using the Flow Jo software (Tree Star, Ashland, OR, USA).
For osteogenic differentiation, 3.1 × 103cells/cm2 of third-passage MSCs were plated on a 10 mm round coverslip in four wells of 24-well plate with MSC basal medium (MSCBM) nutrient medium (Lonza, Walkersville, MD, USA) and were cultured at 37°C with 5% CO2 for 24 h for cell adhesion before undergoing nutrient exchange. The control wells were filled with MSC basal medium (MSCBM), whereas the cells induced to differentiate received hMSC Osteogenic Differentiation Medium (Lonza, Walkersville, USA) every 3 days for 24 days. Calcium deposition was identified by histochemical staining with Alizarin Red S (Sigma-Aldrich, St. Louis, MO, USA). For adipogenic differentiation, third-passage MSCs at a density of 2.1x 104/cm2 were plated in a 24-well plate, on round coverslips as mentioned above. Cells were cultured in MSCBM nutrient medium for 7 days until reaching confluence. Control cultures were kept in MSCGM medium, while adipogenesis was induced by culturing in hMSC Adipogenic Differentiation Medium (Lonza, Walkersville, USA), alternated with MSCGM medium every 3 days for 13 days. Adipogenesis was confirmed using histochemical staining of fat vacuoles with Oil Red O (Sigma-Aldrich, St. Louis, MO, USA).
In vitro differentiation of MSCs into corneal epithelial cells
MSCs were induced to differentiate into corneal epithelial cells by being cultured with CM derived from primary CEC cultures. For the differentiation assay, 3 × 105 second-passage MSCs were seeded in 25 cm2 culture flasks, with nutrient medium for the CEC cultures containing 10% FBS and 40% CM. The cultures were maintained in a humidified incubator with 7% CO2 at 37°C, with the medium changed every 3 or 4 days for 21 days. After this period, cells were phenotyped for pan-cytokeratin antibody conjugated to Alexa 488 (Novus Biologicals, Centennial, CO, USA), anti-cytokeratin 3 antibody (CK3) (Abcam, Cambridge, MA, USA) used with the FITC conjugation kit (Abcam, Cambridge, MA, USA), and anti-cytokeratin 12 antibody (CK12) conjugated to PE (LSBio, Seattle, WA,USA) using flow cytometry (Table 2).
RESULTS
Corneal epithelial cell primary culture techniques
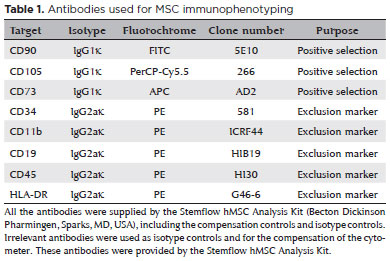
- Culture of explants in flasks without hydrophilic surface treatment: After 14 days of proliferation, only five of the 100 cultures had proliferated, producing only 8 ml of CM. The main issue with this technique was the detachment of the explants during the nutrient medium exchange (Figure 1A).
- Culture of explants in flasks with hydrophilic surface treatment: This culturing technique resulted in the highest yield of CM. Each 75 cm2 culture flask with proliferating CEC from one cornea produced 20 ml of CM until the third cell passage (Figures 1B and 1C).
- Explants on AM: The explants immediately adhered to the AM, with rapid migration and proliferation. However, cellular adhesion to the AM was so intense that the proliferating CEC could not be detached for subsequent cell passages, enabling CM collection only until the first cell confluence (Figure 1D).
- Culture with enzymatic digestion: This technique presented spindle-shaped cell types mixed with epithelial cells, probably representing corneal fibroblasts, which impeded the collection of CM due to culture contamination (Figure 1E).
- Corneal scraping: This technique resulted in high rates of contamination with corneal fibroblasts and cell debris, which makes collecting CM unfeasible (Figure 1F).
Mesenchymal stem cell primary cultures
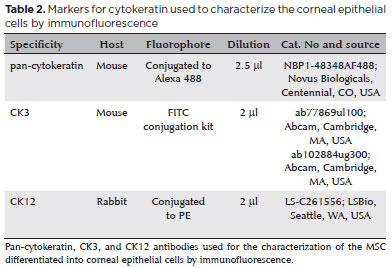
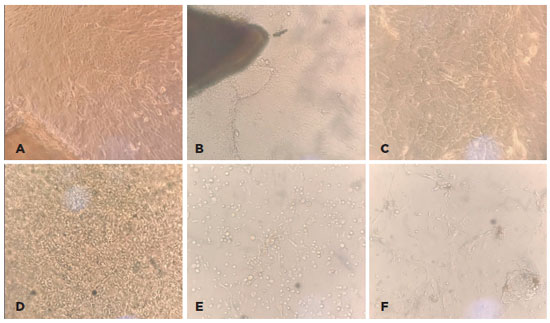
Phenotypic characterization using flow cytometry demonstrated that the analyzed cells were compatible with populations of MSCs, as identified by the characteristics of cell size and granularity. The analyzed cells (Figure 2A) showed high expressions of CD73 (96.1%), CD105 (75.4%), and CD90 (83.5%) and absent or very low expressions of CD34, CD45, CD11b, CD19, and HLA-DR (average, 1.54%). The human MSC population must be highly positive for the surface markers CD73, CD90, and CD105 when measured using flow cytometry. In addition, the markers CD34, CD45, and CD14 must be expressed in less than 2% of the cell population(14); this was used to identify potential contaminants, including hematopoietic stem cells.
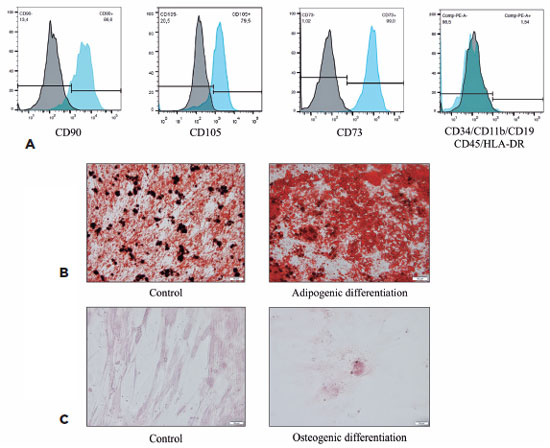
MSCs induced to differentiate into adipocytes were also stained using Oil Red O, showing a large number of lipid vesicles in the cytoplasm. The control cells demonstrated only lipids in plasma membranes (Figure 2B). MSCs induced to differentiate into osteocytes were stained using Alizarin Red S, demonstrating the presence of mineralization nodules of the bone matrix, with calcium deposits. The non-induced control maintained the elongated MSC morphology (Figure 2C).
These results allowed us to validate our approach to ensure the obtaining of a homogeneous culture of human bone marrow MSCs.
MSC differentiation into corneal epithelial cells
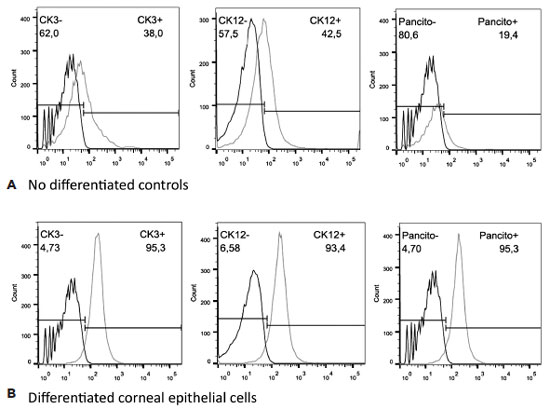
Analysis using flow cytometry showed that MSCs differentiated into CEC, as featured by the expression of corneal cytokeratin-specific markers for CK3 (95.3% of positive cells vs 38% for the control), CK12 (93.4% vs 42.5% for the control), and pan-cytokeratin (95.3% vs 19.4% for the control), confirming the effectiveness of the differentiation protocol (Figure 3).
DISCUSSION
This study demonstrated that the CEC culture technique using explants in flasks with hydrophilic treatment allowed for rapid and strong explant adherence, with the highest success rate in establishing primary cultures and the highest yield of CM.
Current regenerative medicine has been focused on cell-based strategies, and many previous studies have revealed that cell-based therapy is the most common approach available to replace damaged CEC and regenerate the ocular surface(15,16), with efforts on the development of scaffolds for the cell transference therapy. Bone marrow MSCs are a source of cells with high proliferative capacity that can differentiate into lineages of mesenchymal tissues. However, for the differentiation of MSCs into specialized cells, such as CEC, it requires a reasonable amount of CM. The knowledge of the best technique for the primary culture of CEC to obtain CM is of fundamental importance.
The culture technique of explants in flasks without hydrophilic surface treatment did not allow for the sufficient adherence of tissue fragments to the flasks, which detached during the nutrient medium exchanges. This feature resulted to a very low success rate, making its use unfeasible for the production of CM.
The culture using enzymatic digestion and corneal scraping was shown to be unfeasible due to corneal fibroblast contamination. As the MSCs will be differentiated into CEC by the CM obtained from these cultures, contamination by other cell types is unacceptable.
The technique on AM resulted in rapid and strong explant adherence, confirming that cell adherence and proliferation are easily facilitated by the presence of a basal membrane. However, this technique only allowed CM collecting during the first cell confluence, due to the difficulty in detaching the cells from the AM. If there is no need to detach the cells for further cell passages, as in cases of cell transference using AM as scaffold, this is a very fast and easy execution technique.
The culture of explants in flasks with hydrophilic surface treatment allowed a strong adherence of explants, which did not detach during the nutrient medium exchanges, as seen in cultures in flasks without surface treatment. It also resulted in a high yield of CM, as it was collected until the third cell passage.
The characterization of MSCs before the induction of differentiation showed that these cells strongly expressed CD73 (96.1%), CD105 (75.4%), and CD90 (83.5%) and showed an absence or very low levels of CD34, CD45, CD11b, CD19, and HLA-DR (average, 1.54%). The human MSC population must be highly positive for the surface markers CD73, CD90, and CD105, when measured using flow cytometry. Additionally, the markers CD34, CD45, and CD14 must be expressed in less than 2% of the cell population(14). Staining using Oil Red O and Alizarin Red S showed that these cells were able to differentiate into adipocytes and osteoblasts. This ability to differentiate into adipocytes and osteoblasts combined with the immunophenotyping results allowed us to characterize the cells as human bone marrow MSCs.
After the MSC differentiation and induction into corneal epithelial cells using the CM, the flow cytometry analysis revealed that the induced cells were CEC, as demonstrated by the expression of corneal cytokeratin-specific markers for CK3 (95.3% of positive cells vs 38% for the control), CK12 (93.4% vs 42.5% for the control), and pan-cytokeratin (95.3% vs 19.4% for the control), confirming the effectiveness of the cell differentiation protocol.
This study also demonstrated that the in vitro differentiation of bone marrow MSCs into CEC can be achieved using CEC nutrient medium supplemented with 40% CM. Other authors(17) used CM at a 1:1 proportion.
Our results indicated that culturing of explants in flasks with hydrophilic surface treatment is the most suitable technique to obtain CM collected from primary CEC cultures. Despite that the CM can be useful to induce the differentiation of MSCs into CEC, further studies are needed to confirm the utility of this technique to produce tissues for corneal regeneration.
The best CEC culture technique to obtain CM to induce the differentiation of MSCs into corneal epithelial cells is the culture of explants in culture flasks with hydrophilic surface treatment. This technique produces a high yield of CM, which can be useful to obtain corneal epithelial cultured cells from MSCs.
ACKNOWLEDGMENTS
This study was supported by the Sao Paulo State Research Foundation -FAPESP, under grant no. 2015/10727-0. MMHV was supported by a fellowship from the UNESP Research Rectory under grant no. 169.
REFERENCES
1. Mobaraki M, Abbasi R, Omidian Vandchali S, Ghaffari M, Moztarzadeh F, Mozafari M. Corneal repair and regeneration: current concepts and future directions. Front Bioeng Biotechnol. 2019;7:135.
2. Du Y, Funderburgh ML, Mann MM, SundarRaj N, Funderburgh JL. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23(9):1266-75.
3. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71-4.
4. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143-7.
5. Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103(5):697-705.
6. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113(12):1701-10.
7. Gu S, Xing C, Han J, Tso MO, Hong J. Differentiation of rabbit bone marrow mesenchymal stem cells into corneal epithelial cells in vivo and ex vivo. Mol Vis. 2009;15:99-107.
8. Jiang TS, Cai L, Ji WY, Hui YN, Wang YS, Hu D, et al. Reconstruction of the corneal epithelium with induced marrow mesenchymal stem cells in rats. Mol Vis. 2010;16:1304-16.
9. Reinshagen H, Auw-Haedrich C, Sorg RV, Boehringer D, Eberwein P, Schwartzkopff J, et al. Corneal surface reconstruction using adult mesenchymal stem cells in experimental limbal stem cell deficiency in rabbits. Acta Ophthalmol. 2011;89(8):741-8.
10. Liu H, Zhang J, Liu CY, Hayashi Y, Kao WW. Bone marrow mesenchymal stem cells can differentiate and assume corneal keratocyte phenotype. J Cell Mol Med. 2012;16(5):1114-24.
11. Rohaina CM, Then KY, Ng AM, Wan Abdul Halim WH, Zahidin AZ, Saim A, et al. Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Transl Res. 2014;163(3):200-10.
12. Park SH, Kim KW, Chun YS, Kim JC. Human mesenchymal stem cells differentiate into keratocyte-like cells in keratocyte-conditioned medium. Exp Eye Res. 2012;101:16-26.
13. Ramakrishnan A, Torok-Storb B, Pillai MM. Primary marrow-derived stromal cells: isolation and manipulation. Methods Mol Biol. 2013;1035:75-101.
14. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7.
15. Nosrati H, Abpeikar Z, Mahmoudian ZG, Zafari M, Majidi J, Alizadeh A, et al. Corneal epithelium tissue engineering: recent advances in regeneration and replacement of corneal surface. Regen Med. 2020;15(8):2029-44.
16. Mahdavi SS, Abdekhodaie MJ, Mashayekhan S, Baradaran-Rafii A, Djalilian AR. Bioengineering approaches for corneal regenerative medicine. Tissue Eng Regen Med. 2020;17(5):567-93.
17. Blazejewska EA, Schlötzer-Schrehardt U, Zenkel M, Bachmann B, Chankiewitz E, Jacobi C, et al. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27(3):642-52.
Approved by the following research ethics committee: Faculdade de Medicina de Botucatu (#1.376.415).
Disclosure of potential conflicts of interest: All of the authors have no potential conflicts of interest to declare.