

Alparslan Sahin1; Fatih Mehmet Türkcü1; Yasin Çinar2
DOI: 10.5935/0004-2749.2022-0243
To the Editor,
Central serous chorioretinopathy (CSCR) is characterized by fluid acumination in the subretinal space. Several risk factors such as pregnancy, type A personality, smoking, psychological stress, and medications are associated with CSCR(1). Topiramate is an antiepileptic drug that is also used for migraine prophylaxis. Its ocular side effects includes myopic shift, acute angle-closure glaucoma, and choroidal effusion(2). Anterior segment complications of topiramate are more frequent than posterior ocular involvement. However, cases with CSCR-like findings have been reported recently(3,4). Herein, we present a case of CSCR that developed after topiramate treatment that resolved following drug cessation.
A 22-year-old female patient was admitted to the ophthalmology clinic with a complaint of loss of central vision. Her visual acuity was 7/10 bilateral in the Snellen chart. Her intraocular pressure was 15/15 mmHg. Anterior segment examination was unremarkable. Dilated fundus examination revealed bilateral apparent serous elevation of the central retina and pigment epithelial alterations. Optical coherence tomography (OCT) images revealed bilateral central serous detachment of the macula (Figures 1A-B). She had a history of headache, and she was on a 50 mg topiramate (Topamax; Ortho-McNeil Neurologics, Titusville, NJ) treatment twice a day for her complaint for 3 weeks. We considered possible serous detachment of the macula secondary to topiramate treatment, and we discontinued topiramate medication. We did not perform fluorescein angiography because the patient declined it. Additionally, topical treatment of brinzolamide 1% (Azopt) twice a day and nepafenac 0.1% (Nevanac, Alcon Labs, Fort Worth, TX, USA) four times a day were given.
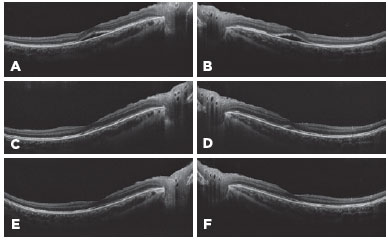
One month later, her visual acuity increased to 8/10 bilaterally. Fundus examination showed retinal pigment epithelial alterations. OCT demonstrated minimal subretinal fluid (Figure 1C-D). She continued the topical eye drops. Two months after the first examination, her visual acuity improved to 10/10 bilaterally. Fundus examination was unremarkable, and OCT images revealed normal anatomy of the retina (Figure 1E-F).
Several medications such as steroids, phosphodiesterase inhibitors, etc., are risk factors for CSCR development(1). This case revealed that topiramate may cause CSCR that resolved after medication cessation. Although anterior segment side effects have been frequently reported, topiramate may also affect the posterior segment of the eye. Rosenberg et al. reported two cases of macular neurosensory retinal detachment secondary to topiramate use(4). One patient had bilateral, and the other had unilateral serous detachment. However, their case with bilateral involvement was on topical steroids during the symptoms. In addition, Mazumdar et al. reported a case of unilateral serous retinal detachment(3). Additionally, their case was under topical steroid treatment. These case reports showed that cessation of topiramate leads to the recovery of serous detachment.
Clinicians should consider the possible effects of topiramate treatment on the pathogenesis of CSCR. Topiramate cessation may result in the regression of the serous detachment of the retina. Since a prompt diagnosis is important to prevent further damage to the retina, both ophthalmologists and neurologists should be aware of serous retinal detachment after topiramate treatment.
REFERENCES
1. Liu B, Deng T, Zhang J. Risk factors for central serous chorioretinopathy: a systematic review and meta-analysis. Retina. 2016; 36(1):9-19.
2. Ozturk BT, Genc E, Tokgoz M, Kerimoglu H, Genc BO. Ocular changes associated with topiramate. Curr Eye Res. 2011;36(1):47-52.
3. Mazumdar S, Tripathy K, Sarma B, Agarwal N. Acquired myopia followed by acquired hyperopia due to serous neurosensory retinal detachment following topiramate intake. Eur J Ophthalmol. 2019; 29(1):NP21-4.
4. Rosenberg K, Maguire J, Benevento J. Topiramate-induced macular neurosensory retinal detachment. Am J Ophthalmol Case Rep [Internet]. 2017[cited 2022 Jan 21];7:31-7. Available from: Topiramate-induced macular neurosensory retinal detachment - PMC (nih.gov)
Submitted for publication:
July 4, 2022.
Accepted for publication:
July 30, 2022.
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.