

Oswaldo Ferreira Moura Brasil1,2; Mariana Batista Gonçalves1; Felipe Muralha1; Rodrigo M Navarro1; Bruno de Queiroz Alves1; Mariana Kawamuro1; Emmerson Badaró1; Mauricio Maia1,3
DOI: 10.5935/0004-2749.20190101
ABSTRACT
PURPOSE: The aim of this study was to determine the functional and anatomical success rates as well as the safety of sutureless combined surgery involving vitreous base removal and internal limiting membrane peeling after Brilliant Blue G (0.5 mg/mL) staining for the management of idiopathic macular holes after three years.
METHODS: Forty-six eyes of 46 patients with an idiopathic macular hole were enrolled in this retrospective study. The inclusion criteria were macular holes with a minimum linear diameter below 1,500 mm, 0.05 or better decimal best-corrected visual acuity and duration of symptoms less than two years. The exclusion criteria included pregnancy, optic nerve atrophy, advanced glaucoma, and other chronic ocular diseases. The surgical procedure included internal limiting membrane peeling after Brilliant Blue G (0.5 mg/mL) staining, along with C3F8 tamponade and face-down positioning for three days postoperatively. Ophthalmologic examinations and optical coherence tomography were performed at 1 and 7 days and 1, 6, 12, 24, and 36 months postoperatively. If no anatomic closure of the macular holes occurred within the first month, the area of the internal limiting membrane peeling was enlarged in a second procedure. Multiple logistic regression and chi-squared tests were used for data analyses, and p-values of <0.05 were considered significant.
RESULTS: Out of 46 eyes with a preoperative idiopathic macular hole, anatomic closure was achieved in 42 (91.3%) after one procedure and in 45 (97.8%) after an additional surgery. The median postoperative best-corrected visual acuity improvement was 0.378 (range: 0.050-0.900) decimal. None of the patients experienced macular hole reopening, surgery-related complications, or ocular complications related to the dye.
CONCLUSION: Combined surgery including vitreous base removal and internal limiting membrane peeling after staining with Brilliant Blue G (0.5 mg/mL) for the management of idiopathic macular holes resulted in adequate staining, best-corrected visual acuity improvement, and macular hole closure with no signs of ocular toxicity at the three-year follow-up examination.
Keywords: Vitrectomy/methods; Macular hole; Coring agents; Rosaniline dyes; Brilliant blue
RESUMO
OBJETIVO: Determinar, após 3 anos de seguimento, as taxas de sucesso funcional e anatômico e a segurança da cirurgia combinada sem sutura, incluindo remoção da base vítrea e da membrana limitante interna após coloração com azul brilhante (0,5 mg/ml) para o manejo de buracos maculares idiopáticos.
MÉTODOS: Quarenta e seis olhos de 46 pacientes com buraco macular idiopático foram incluídos neste estudo retrospectivo. Os critérios de inclusão foram: buraco macular com diâmetro linear mínimo menor que 1500 micrômetros, acuidade visual com melhor correção de 0,05 decimal ou melhor e tempo de sintomas menor que 2 anos. Os critérios de exclusão foram gravidez, atrofia do nervo óptico, glaucoma avançado ou outra doença ocular crônica. A técnica cirúrgica incluiu a remoção da membrana limitante interna após coloração com Azul Brilhante 0,5 mg/ml, tamponamento com C3F8 posicionamento em prona ção durante 3 dias de pós-operatório. O seguimento foi realizado por exame oftalmológico e Tomografia de Coerência Óptica no 1 e 7 dias, 1, 6, 12, 24 e 36 meses de pós-operatório. Se o fechamento anatômico do buraco macular não fosse atingido na visita de um mês, realizava-se um segundo procedimento no qual a área do peeling da membrana limitante interna era ampliada. Para análise estatística, foram utilizados testes de regressão logística múltipla e Qui-quadrado. Valores de p menores que 0.05 foram considerados estatisticamente significativos.
RESULTADOS: Dos 46 olhos com buraco macular idiopático, 42 (91,3%) obtiveram fechamento do buraco macular após um procedimento cirúrgico e 45 (97,8%) após uma cirurgia adicional. A média de melhora da acuidade visual com melhor correção no pós-operatório foi de 0.378 (0.050-0.900) decimal. Não foram observados: reabertura do buraco macular, complicações relacionadas ao procedimento cirúrgico ou complicações relacionadas ao corante.
CONCLUSÃO: A cirurgia combinada sem sutura que incluiu remoção da base vítrea e remoção membrana limitante interna após coloração com Azul Brilhante (0,5 mg/ml) para o tratamento de buracos maculares idiopáticos foi realizada com adequada capacidade de coloração, melhora da acuidade visual e fechamento do buraco macular sem sinais de toxicidade ocular no seguimento de 3 anos.
Descritores: Vitrectomia/métodos; Buraco macular; Corantes; Corantes de rosanilina; Azul brilhante
INTRODUCTION
Macular holes (MHs) are characterized by a total-thickness defect of the neurosensory retina in the fovea1,2. The prevalence of this pathology varies widely, ranging from 0.2 cases/1,000 inhabitants in the Blue Mountains Study to 3.3 cases/1,000 inhabitants in the Baltimore Eye Study2. A population-based study conducted in the United States demonstrated an incidence of 7.8 cases/100,000 inhabitants annually2. This disease occurs more frequently in women at a ratio of 3:1 compared to men, especially after 65 years of age, and 10% of the cases are bilateral3-5.
Although the pathogenesis of MH is not fully understood, vitreoretinal traction due to incomplete detachment of the posterior vitreous may cause foveal cyst formation, which can culminate in the formation of MHs if the traction is maintained. Tangential vitreoretinal tensile forces and involutional changes in the retinal layers are also implicated in the pathogenesis of the disease2,4.
Several classifications of MHs exist. According to the Gass classification, MHs present in the following stages: (1) impending MH, (1A) a yellowish spot on the fovea, and (1B) a yellowish ring. The total-thickness retinal defect is absent in Stage 1, and fundoscopic changes result from vitreoretinal traction and subfoveal cysts. Stage 2 is characterized by a total-thickness defect less than 400 mm in diameter, Stage 3 is characterized by a total-thickness retinal defect that exceeds 400 mm in diameter without a Weiss ring, and Stage 4 corresponds to Stage 3 after complete detachment of the posterior vitreous and the presence of a Weiss ring2.
In 2013, the International Vitreomacular Traction Study (IVTS) proposed a new classification for MHs based on optical coherence tomography (OCT) findings and MH size (i.e., small, <250 mm; medium, 250-400 mm; and large, >400 mm). The presence or absence of vitreomacular traction (VMT) and primary or secondary holes resulting from VMT and related to, for example, high myopia, blunt trauma, and macular schisis was also included in the classification1.
Surgery for MH repair typically consists of pars plana vitrectomy (PPV), separation and removal of the posterior vitreous cortex, removal of the internal limiting membrane (ILM), injection of gas or air into the vitreous cavity, and maintenance of a face-down position postoperatively6. According to recent studies, the success rate for MH surgery currently exceeds 90%, a vast improvement over the 60% success rate reported when the surgical technique was first developed1,5,7.
Chromovitrectomy refers to the use of dyes to facilitate the visualization of thin, transparent, intraocular tissues during vitrectomy8. Numerous dyes can be used as aids for ILM peeling, such as Indocyanine Green (ICG), Infracyanine Green, and Brilliant Blue G (BBG). Among these, BBG is the most widely used for ILM staining globally because of its high affinity for the ILM and good safety profile at a concentration of 0.25 mg/mL9. Attempts were recently made to further improve ILM staining, including by increasing the dye concentration10.
The objective of the current study was to determine the functional and anatomic success rates, as well as the safety of sutureless combined surgery involving vitreous base removal and ILM peeling after staining with BBG (0.5 mg/mL) for the management of idiopathic MHs after three years of follow-up.
METHODS
Patients
This retrospective study included 46 eyes of 46 patients with idiopathic MH evaluated at the vitreoretinal disease units of two Brazilian centers. The Ethics Committee of the Federal University of São Paulo approved this study, which was conducted according to the research guidelines of the Association for Research in Vision and Ophthalmology and the tenets of the Declaration of Helsinki. All patients provided informed consent regarding the benefits and risks of the surgical procedure as well as the concentration of the dye tested.
Inclusion and exclusion criteria
Patients of both genders that met the following criteria were included: being above 18 years of age, duration of symptoms less than two years, MHs with a minimum linear diameter of less than 1,500 mm, and 0.05 or better best-corrected visual acuity (BCVA) in decimal. The exclusion criteria were pregnancy, presence of optic nerve atrophy, advanced glaucoma, or other chronic ocular diseases.
Physical evaluation, ocular examinations, and follow-up
All patients underwent a physical evaluation preoperatively that included measurement of the systemic blood pressure and heart rate. An ocular examination was performed preoperatively and 1 and 7 days and 1, 6, 12, 24, and 36 months postoperatively. This examination included BCVA measurements using a Snellen chart, intraocular pressure measurements using Goldmann tonometry, and slit-lamp and fundus evaluations. All BCVA measurements were converted to decimal equivalents of Snellen BCVA for analysis. Spectral-domain OCT was also performed using a Spectralis device (Heidelberg Engineering, Heidelberg, Germany).
Surgical procedure
Two surgeons (OFMB and MM) experienced in chromovitrectomy and in using binocular indirect ophthalmic microscopy (System; Oculus, Wetzlar, Germany) for noncontact, wide-angle, vitreous surgery performed all the standardized surgical procedures. Nuclear cataracts frequently develop after vitrectomy; therefore, combined phacovitrectomy was performed in all phakic eyes using a 2.4 mm clear corneal incision with implantation of an aspheric Akreos AO intraocular lens (Bausch & Lomb, Rochester, NY, USA) or an AcrySof IQ intraocular lens (Alcon, Fort Worth, TX, USA). The incision was left unsutured at the end of phacoemulsification. Four-port PPV using three 23-gauge valved trocars was performed, and a fourth sclerotomy using a 25-gauge incision was created followed by insertion of a chandelier light pipe Tornambe (Synergetics, O’Fallon, MO, USA) connected to a Photon II light source (Synergetics). Surgery was performed using both the Constellation vitrectomy system (Alcon) and the Stellaris PC vitrectomy system (Bausch & Lomb). Core vitrectomy was performed at a rate of 5,000 cuts/min with 200 mmHg aspiration power.
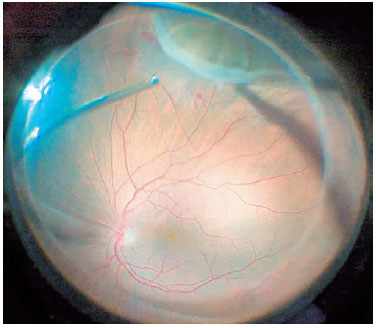
For cases in which the posterior hyaloid was attached to the posterior pole, the membrane was stained by injecting 0.1 mL of triamcinolone acetonide into the vitreous cavity and detached using a vitreous probe aspirated at a rate of 300-400 mmHg with the cutting mode turned off. The surgeon shaved the vitreous base while performing an indentation maneuver with his other hand (Figure 1). In all cases, we aimed to remove the entire vitreous base using 5,000 cuts/min with 200 mmHg aspiration power.
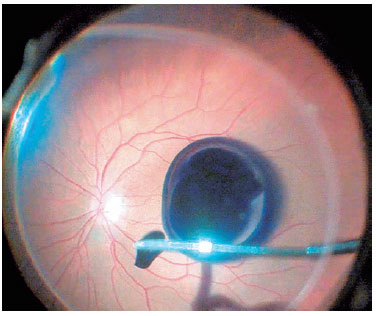
After the vitreous was completely removed, 0.3 mL of 0.05% BBG was flushed through the posterior pole using a 23-gauge soft-tip cannula, which was subsequently disconnected carefully from the infusion system to avoid injection directly into the hole (Figure 2). The BBG injected had a concentration of 0.5 mg/mL, osmolarity of 280 mOsm, density of 1.1, pH of 7.00, and an intense dark-blue color.
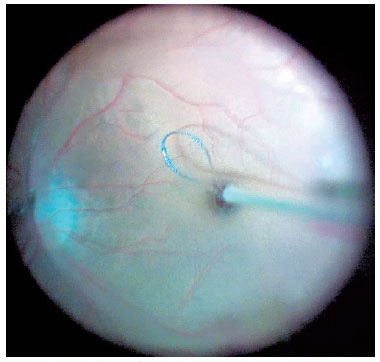
For macular surgery, a Machemer contact lens (Volk, Cleveland, OH, USA) was used, and the ILM was peeled using 23-gauge intraocular forceps. Peeling was initiated by grasping the ILM over the inferior macular region with the forceps or using a Finesse™ flex loop to create an ILM flap (Figure 3), and then it was extended in a circum ferential manner over the area, including the macula, without touching the retinal surface (Figure 4).
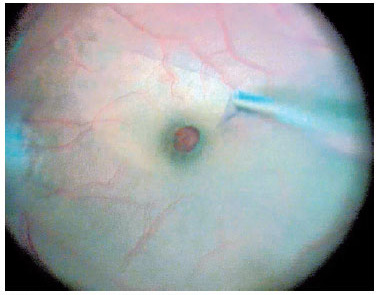
At the end of the surgery, complete removal of the dye was attempted; the valved trocars and the chandelier light pipe were removed, and no sutures were placed. Octafluoropropane 15% (C3F8) was injected into the vitreous cavity as a vitreous substitute. A subconjunctival injection of 0.2 mL of a gentamicin and dexamethasone solution was administered before the lid speculum was removed. The patients were instructed to maintain a face-down prone position for three days postoperatively.
A second vitrectomy was performed if no MH closure occurred within 30 days postoperatively. In this second procedure, the area of ILM peeling was enlarged after BBG staining. C3F8 was also used and the patients were instructed to maintain a face-down position for seven days postoperatively.
Statistical analysis
Multiple logistic regression analysis was performed to determine the likelihood of a positive increase in BCVA as a function of the demographics and preoperative variables. Relationships were considered significant if <0.05. The association between the MH minimum linear diameter and the percentage of patients with improved BCVA was tested using the chi-squared test. The analyses were performed using SPSS v15.0 software (IBM Corp., Armonk, NY, USA).
RESULTS
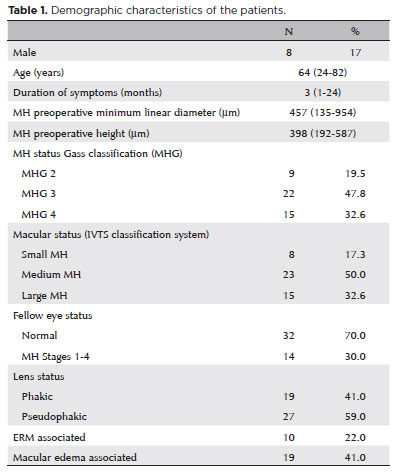
Clinical findings
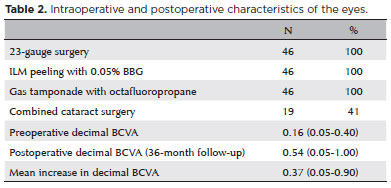
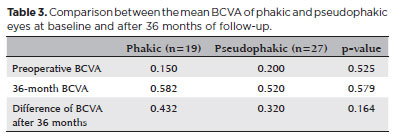
The average age of the 46 patients (38 women, 8 men) was 64 years (range: 24-82 years). According to the IVTS Group Classification of Vitreomacular Adhesion, Traction, and Macular Hole1, 8 eyes had small MHs, 23 had medium MHs, and 15 had large MHs (Table 1). Anatomic closure was achieved in 42 (91.3%) eyes after one procedure and in 45 (97.8%) eyes after the second surgery. The mean BCVA was 0.16 (range: 0.05-0.40) decimal preoperatively and 0.54 (range: 0.05-1.00) decimal after 36 months, with a median postoperative BCVA improvement of 0.37 (range: 0.05-0.90) decimal (Table 2). BCVA improved in all eyes, and the patients were followed up for at least 36 months (range: 24-81 months). A subgroup analysis comparing the BCVA improvements in phakic and pseudophakic patients showed that the former group achieved better results, although this finding was not statistically significant (p=0.579). This result is expected once cataract surgery itself can improve the BCVA (Table 3).
Intraoperative findings
Two experienced surgeons performed all surgeries (i.e., phacovitrectomy for 19 eyes and vitrectomy only for the remaining pseudophakic eyes). All surgeries were performed as described previously. 0.05% BBG satisfactorily stained the ILMs after being flushed through the posterior pole.
Follow-up
Three years postoperatively, no dye toxicity was observed during clinical examinations. The MH did not close within 30 days after the first procedure in four patients; therefore, a second procedure was performed as described previously using the same dye, with C3F8 and seven days of face-down positioning postoperatively. This procedure differed from the first in that the area of ILM peeling was enlarged and the patient was instructed to spend more days in the prone position. MH closure was achieved in three of these four patients. No MHs reopened in this series, and no retinal detachment or other complications developed as a result of the vitrectomy at the three-year follow-up examination.
DISCUSSION
All phakic eyes in the current study underwent phacovitrectomy. This is an important surgical step because most patients with MH are elderly. Vitrectomy alone has a high rate of cataract induction, and isolated cataract surgery after vitrectomy carries a risk of MH reopening as well as a second surgery11. Although the intraoperative use of triamcinolone and its presence during the follow-up period might lead to delayed closure and reopening of MH12,13, this did not occur in our study.
During the PPV, posterior hyaloid detachment was performed in all patients in whom the detachment did not occur naturally. This procedure is important mainly for MH Stages 2 and 3 of the Gass classification, in which the vitreous is still attached to the operculum and disk (Stage 2) or only to the disc (Stage 3). Afterward, the surgeon performed an indentation maneuver to remove the vitreous base. Removal of as much vitreous as possible is important because it enables the injection of a larger gas volume into the eye, which increases the tamponade effect and prevents gas bubbles from exerting traction in the residual areas of the vitreous, which can increase the probability of retinal tears and detachment2.
After vitreous removal, the ILM was stained with BBG and peeled. Eckardt first proposed ILM peeling in 1997 as a way to improve the success rate of MH surgery14. Despite being initially mistrusted, the technique has gained increasing acceptance among ophthalmologists and is currently a standard surgical practice5,9,10. In a meta-analysis performed by Rahimy et al. analyzed 5,480, the authors assessed 5,480 MH surgeries reported in 50 publications and concluded that ILM peeling significantly reduced the rates of MH reopening5. They suggested that removing the ILM, which serves as a support for cellular growth, prevents the formation of ERMs that cause traction and reopening of the hole. Peeling also causes mechanical trauma, inducing the release of cytokines, which contributes to the healing of the retinal defect2,5.
Among the dyes used for staining and removal of ILMs, BBG has good affinity for the structure and a good safety profile. BBG is also more water-soluble than other dyes used for this purpose, such as ICG. Thus, BBG penetrates less into the cells and is more easily washed from the eye8.
Recent attempts have been made to improve ILM staining, for example, using heavy substances to increase contact between the dye and the posterior pole (deuterium oxide and 10% dextrose)15-17, air-fluid exchange18, and increased dye concentration10. The main disadvantage of air-fluid exchange is the increased risk of surgical complications, such as subretinal migration of the dye through the MH and retinal tears19. In order to avoid air-fluid exchange, we propose applying a stronger dye concentration with good staining ability for the ILM to the retinal surface in a fluid-filled eye and disconnecting the infusion line during injection.
In the present study, we performed vitrectomy with ILM peeling using BBG at a higher concentration than usual, with comparable success rates and neither ocular complications nor toxicity. The success rates for anatomical closure were 91.3% in 42 out of 46 eyes after the first surgery and 97.8% in 45 out of 46 eyes after the second surgery, which is consistent with previous reports5,20. If a second procedure was necessary, the ILM peeling area was enlarged using the same 0.05% BBG dye, with C3F8 tamponade and face-down positioning postoperatively for seven days.
The mean preoperative BCVA was 0.162 decimal, which improved to 0.540 decimal after three years of follow-up (Table 2). The BCVA did not decrease in any patient. The visual outcomes were significantly (p<0.05) better in patients with small MHs than in those with large MHs. The improvement of the visual acuity coupled with the fact that no abnormalities in the retinal pigment epithelium were observed in the fundoscopy and OCT greatly reduced the likelihood of dye toxicity. However, additional studies using different methods to evaluate the dye toxicity are necessary for a definitive conclusion.
During the three-year follow-up, no MHs reopened and no retinal detachment or other complications developed as a result of the vitrectomy. The finding of no retinal detachments at the three-year follow-up examination is promising; we hypothesized that this might have been related to the shaving of the vitreous base and meticulous care regarding possible tear formation, especially at 6 h position.
The strengths of the current study were the novel concentration of BBG used (0.5 mg/mL), the good safety profile, the satisfactory number of patients, and the long follow-up period during which the eyes that underwent four-port phacovitrectomy associated with vitreous base shaving were evaluated. No postoperative retinal detachment was detected in any patient at the three-year follow-up evaluation. The limitations of this study were its retrospective design, the fact that two surgeons performed the procedures, the absence of a control group, and the small number of eyes included.
In conclusion, ILM peeling using 0.5 mg/mL BBG during 23-gauge sutureless four-port PPV with vitreous base shaving resulted in adequate staining of the ILMs, a high success rate of idiopathic MH closure, BCVA improvement, and no retinal tears/detachment at the three-year follow-up examination. Clinical trials should be undertaken with more eyes and controls to confirm the current findings and demonstrate the advantages of this surgical technique compared to three-port PPV and no shaving of the vitreous base.
REFERENCES
1. Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The International Vitreomacular Traction Study Group classi¬fication of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611-9.
2. Ryan SJ. Retina 5th ed. New York: Saunders 2013. Gaudric A, Tadayoni R. Macula Hole. Chapter 117, p.1969-75.
3. Ávila M, Lavinski J, Júnior CAM. Série de Oftalmologia Brasileira: Retina e vítreo. 3a ed. Rio de Janeiro: Cultura Médica: Guanabara Koogan, 2013. p. 130.
4. Kanski JJ, Bowling B, editor. Oftamologia clínica. 7th ed. Rio de Janeiro: Elsevier; 2012. p. 629.
5. Rahimy E, McCannel CA. Impact of internal limiting membrane peeling on macular hole reopening: A systematic review and meta-analysis. Retina. 2016;36(4):679-87.
6. Basic and Clinical Science Course, Section 12: Retina and vitreous, 2016-2017. San Francisco (CA): American Academy of Ophthalmology, c2019. p. 294, 336.
7. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654-9.
8. Al-Halafi AM. Chromovitrectomy: update. Saudi J Ophthalmol. 2013; 27(4):271-6.
9. Caiado RR, Filho MN, Maia A, Rodrigues EB, Farah ME, Maia M. State of the art in chromovitrectomy. Rev Bras Oftalmol. 2014;73(6): 363-76.
10. Rodrigues EB, Costa EF, Penha FM, Melo GB, Bottós J, Dib E, et al. The use of vital dyes in ocular surgery. Surv Ophthalmol. 2009; 54(5):576-617.
11. Bhatnagar P, Kaiser PK, Smith SD, Meisler DM, Lewis H, Sears JE. Reopening of previously closed macular holes after cataract extraction. Am J Ophthalmol. 2007;144(2):252-9.
12. Koto T, Inoue M, Shinoda K, Ishida S, Tsubota K. Residual crystals of triamcinolone acetonide in macular hole may prevent complete closure. Acta Ophthalmol Scand. 2007;85(8):913-4.
13. Yamauchi Y, Nakamura H, Hayakawa K, Sawaguchi S. Persistence of triamcinolone acetonide following macular hole surgery. Acta Ophthalmol Scand. 2006;84(5):711-2.
14. Eckardt C, Eckardt U, Groos S, Luciano L, Reale E. [Removal of the internal limiting membrane in macular holes. Clinical and morphological findings]. Ophthalmologe. 1997;94(8):545-51. German.
15. Costa EP, Rodrigues EB, Farah ME, Dib E, Penha F, Magalhães O Jr, et al. Vital dyes and light sources for chromovitrectomy: comparative assessment of osmolarity, pH, and spectrophotometry. Invest Ophthalmol Vis Sci. 2009;50(1):385-91.
16. Haritoglou C, Schumann RG, Kampik A, Gandorfer A. Heavy brilliant blue G for internal limiting membrane staining. Retina. 2011;31(2):405-7.
17. Pelayes DE, Kuhn F, Folgar AM, Takahashi W, Bastien A, Vinicius PN, et al. Staining of the internal limiting membrane with the use of heavy brilliant blue G. Ophthalmic Res. 2012;48(Suppl 1):21-5.
18. Farah ME, Maia M, Rodrigues EB. Dyes in ocular surgery: principles for use in chromovitrectomy. Am J Ophthalmol. 2009;148(3):332-40.
19. Lesnik Oberstein SY, Mura M, Tan SH, de Smet MD. Heavy trypan blue staining of epiretinal membranes: an alternative to infracyanine green. Br J Ophthalmol. 2007;91(7):955-7.
20. la Cour M, Friis J. Macular holes: classification, epidemiology, natural history and treatment. Acta Ophthalmol Scand. 2002; 80(6):579-87.
Approved by the following research ethics committee: Universidade Federal de São Paulo (#645/2016).
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.