

Zelia M Correa1; Rafaela Caixeta Faraj2; Rodrigo Jorge3
DOI: 10.5935/0004-2749.20190104
Individualized management of cancer patients based on predictive molecular testing has sparked the interest of clinicians and medical researchers and is part of important recent changes in overall oncologic patient management. Unfortunately for patients, the field of ocular oncology is not moving at the same pace. For at least a proportion of ocular oncologists, the diagnosis and prognosis of uveal melanoma remain based solely on clinical features(1,2). After nearly 100 years of clinical research, currently known factors associated with metastasis in uveal melanoma include older age at diagnosis, anterior tumor location, larger tumor size (i.e., basal diameter and thickness), epithelioid cell type, and local tumor invasion through the sclera(1-3).
By contrast, sampling of posterior melanocytic uveal tumors using fine-needle aspiration biopsy (FNAB) or vitrectomy cutter choroidal biopsy (VCCB) has been exhaustively researched in the past three decades(4-6). Although several techniques have been reported and explained, there is a lack of uniformity among ocular oncologists that has led to discrepant results in the literature, and these include variable rates of cellular yield and severe complications such as seeding of the biopsy needle track and intraocular hemorrhage(6-9).
For clinicians who were early adopters, the use of FNAB (or VCCB) has become part of the standard of care for assessing-with minimal or calculated risks-the diagnosis and prognosis of posterior uveal melanomas (PUMs) and borderline melanocytic uveal tumors(4,7,10). The benefits of tumor sampling have been shown to outweigh the risks, particularly with respect to the progress of more advanced techniques for detecting chromosomal and genomic alterations, and these have predictive prognostic accuracy superior to that of the aforementioned clinical and pathologic features(11,12). Approximately 15 years ago, multiple centers worldwide adopted the detection of monosomy 3 for metastatic prediction in uveal melanoma. However, essential indicators of the utility of monosomy 3 as a clinical marker of metastatic risk, such as sensitivity and specificity, have not been reported in a prospective multicenter study(11). Multiple recent reports have shown that the molecular classification of uveal melanomas based on gene expression profile (GEP) is a robust indicator of uveal melanoma metastasis and seems to be superior to monosomy 3 and multiplex ligation-dependent probe amplification for individualized patient management(12). Thus far, the GEP test remains the only prognostic assay for uveal melanoma that was prospectively validated in a multicenter trial(13). The ability to obtain this level of information with respect to a particular tumor is rapidly changing the approach to the management of uveal melanoma and allowing oncologists to adjust the frequency of surveillance testing for metastasis. Recent developments have incorporated the preferentially expressed antigen in melanoma (PRAME) protein as a very sensitive marker for metastatic risk in PUM(14,15). PRAME is a tumor-associated antigen that is encoded by the PRAME gene, located on chromosome 22 loci q11.2, and this antigen is recognized by cytolytic T lymphocytes. Interestingly, PRAME is not expressed in normal tissues except the testis. Such fact may have ancestry implications that have not yet been determined. The genetic and genomic information obtained by FNAB is increasingly relevant to individualized management for patients as oncologists are considering targeted therapies for metastatic disease and multiple clinical trials are being launched(14-17).
Despite these developments and related ongoing discussions, the available literature reminds us that many ocular oncologists continue to fear the risk of tumor seeding following uveal melanoma biopsy, and subsequent development of metastasis(9). Ophthalmologists practicing ocular oncology should feel reassured that complications such as seeding of the needle tract, extraocular tumor extension, or spreading related to this procedure are extremely rare, and these complications only occur if the appropriate technique is not used(7,8,10). Multiple researchers have shown that it is feasible to sample small tumors with minimal or no side effects(4-6). In a recently published study involving a large cohort of patients, all-cause and melanoma-specific mortality after primary treatment were similar among biopsied and non-biopsied patients with PUM(9). In addition, the results confirmed that biopsy of PUM was helpful and did not increase metastatic risk. This evidence may address the reservations of many ophthalmologists and encourage them to consider the important indications and applications for FNAB in the assessment of patients with PUM.
Finally, it is important to determine the indications for FNAB in patients with posterior uveal tumors. A posterior uveal tumor is generally biopsied for diagnostic, confirmatory, investigational, and prognostic reasons(4). Diagnostic biopsies are performed in patients with indeterminate tumors, such as small lesions in the nevus versus melanoma category(10) or amelanotic tumors in the melanoma versus metastasis category(18). Figures 1 and 2 show a small melanocytic choroidal tumor in the nevus versus melanoma category that was assessed by diagnostic FNAB because of an increase of subretinal fibrosis during 6 months of follow-up. Cytology examination revealed the tumor to be a nevus, and GEP testing showed that it was Class 1A PRAME-negative. Confirmatory biopsies are performed when, although the clinical diagnosis is certain, the patient or other members of the care team request confirmation, and such biopsies are also performed when necessary for management decisions. Investigational biopsies are performed with approval of an Ethics Committee (or Institutional Review Board) and patient consent for the purpose of developing new surgical techniques, evaluating biopsy yield, or processing/testing tissue.
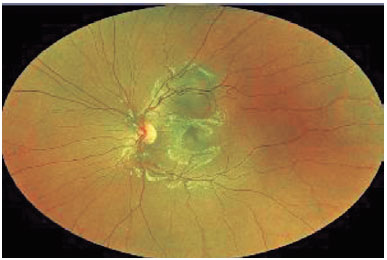
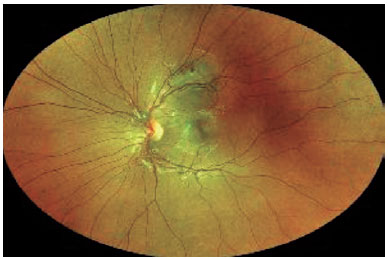
Finally, prognostic biopsies are currently performed in most patients with PUM in order to assess the patient’s risk of metastatic disease(7,11). The clinical value of a clear indication for biopsy is underestimated since in our experience, these indications will determine how the tumor sample will be obtained and tested, if or how it will be used to guide patient management, and what frequency of surveillance testing is needed(19).
In summary, biopsy using either FNAB or VCCB should be included in the assessment of patients with PUM because of its ability to confirm (or distinguish) tumor type via cytology, as well as its ability to inform prognosis.
Thus, biopsy can guide systemic surveillance for metastasis. The lack of consensus in determining indications, biopsy techniques, testing, and processing of biopsy samples has been a barrier to the broad adoption of FNAB as an evaluation component for patients with PUMs.
REFERENCES
1. Gass JD. Problems in the differential diagnosis of choroidal nevi and malignant melanoma. XXXIII Edward Jackson Memorial lecture. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83:19-48.
2. Char DH, Miller T. Accuracy of presumed uveal melanoma diagnosis before alternative therapy. Br J Ophthalmol. 1995;79:692-6.
3. Augsburger JJ, Gamel JW. Clinical prognostic factors in patients with posterior uveal malignant melanoma. Cancer. 1990;66:1596-600.
4. Augsburger JJ, Shields JA. Fine needle aspiration biopsy of solid intraocular tumors: indications, instrumentation and techniques. Ophthalmic Surg. 1984;15:34-40.
5. Char DH, Miller TR, Ljung BM, Howes EL Jr, Stoloff A. Fine needle aspiration biopsy in uveal melanoma. Acta Cytol. 1989;33:599-605.
6. Finn AP, Materin MA, Mruthyunjaya P. CHOROIDAL TUMOR BIOPSY: A Review of the Current State and a Glance Into Future Techniques. Retina. 2017 Dec 26. doi: 10.1097/IAE.0000000000001997. [Epub ahead of print]
7. Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):131-135.
8. Schefler AC, Gologorsky D, Marr BP, Shields CL, Zeolite I, Abramson DH. Extraocular extension of uveal melanoma after fine-needle aspiration, vitrectomy, and open biopsy. JAMA Ophthalmol. 2013; 131:1220.
9. Bagger M, Smidt-Nielsen I, Andersen MK, Jensen PK, Heegaard S, Andersen KK, Friis S, Kiilgaard JF. Long-Term Metastatic Risk after Biopsy of Posterior Uveal Melanoma. Ophthalmology. 2018 Apr 25. pii: S0161-6420(17)33868-X. doi: 10.1016/j.ophtha.2018.03.047. [Epub ahead of print]
10. Augsburger JJ, Corrêa ZM, Schneider S, et al. Diagnostic transvitreal fine-needle aspiration biopsy of small melanocytic choroidal tumors in nevus versus melanoma category. Trans Am Ophthalmol Soc. 2002;100:225-32-4.
11. Correa ZM. Assessing Prognosis in Uveal Melanoma. Cancer Control. 2016;23(2):93-8.
12. Worley LA, Onken MD, Person E, Robirds D, Branson J, Char DH, Perry A, Harbour JW. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res. 2007;13(5):1466-71.
13. Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596-603.
14. Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an Independent Biomarker for Metastasis in Uveal Melanoma. Clin Cancer Res. 2016;22(5):1234-42.
15. Field MG, Durante MA, Decatur CL, et al. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in Class 1 and Class 2 uveal melanomas. Oncotarget. 2016;7(37):59209-19.
16. Gezgin G, Luk SJ, Cao J, et al. PRAME as a Potential Target for Immunotherapy in Metastatic Uveal Melanoma. JAMA ophthalmology. 2017;135(6):541-9.
17. Yang J, Manson DK, Marr BP, Carvajal RD. Treatment of uveal melanoma: where are we now? Ther Adv Med Oncol. 2018 Feb 21;10:1758834018757175.
18. Augsburger JJ. Fine needle aspiration biopsy of suspected metastatic cancers to the posterior uvea. Trans Am Ophthalmol Soc. 1988; 86:499-560.
19. Corrêa ZM, Augsburger JJ. Indications for Fine Needle Aspiration Biopsy of Posterior Segment Intraocular Tumors. Am J Ophthalmol. 2019 Jun 3. pii: S0002-9394(19)30250-8. doi: 10.1016/j.ajo.2019. 05.018. [Epub ahead of print]
Submitted for publication:
July 20, 2019.
Accepted for publication:
August 16, 2019.
Funding: This study received no specific financial support.
Disclosure of potential conflicts of interest: Dr. Correa is a consultant for Castle Biosciences, Inc., but has not received any compensation to write this article; Dr Faraj and Dr Jorge do not have any conflicts.