

Murat Gunay1; Gokhan Celik2; Elvin Yildiz3; Handan Bardak3; Heves Kirmizibekmez4; Nermin Koc5; Betul Onal Gunay6
DOI: 10.5935/0004-2749.20190056
ABSTRACT
Purpose: We aimed to assess ocular surface characteristics in children with Hashimoto’s thyroiditis without thyroid-associated ophthalmopathy and compare the results with those of healthy children.
Methods: Twenty-two children with Hashimoto’s thyroiditis (Group 1) and 20 healthy children without any ocular and/or systemic disorder (Group 2) were enrolled in the study. Ocular Surface Disease Index questionnaire, tear film osmolarity measurement (TearLab Osmolarity System, San Diego, CA, USA), Schirmer and tear film breakup time tests, meibography, and conjunctival brush cytology were performed and compared the results between the groups.
Results: The study group included 19 girls and 3 boys in Group 1 and 12 girls and 8 boys in Group 2 (p=0.081). Thyroid-associated ophthalmopathy was not identified in any of the patients. Mean tear film osmolarity was 310.23 ± 11.98 mOsm/l in Group 1 and 313.60 ± 15.03 mOsm/l in Group 2 (p=0.424). Mean Schirmer test score was lower in Group 1 (14.91 ± 6.27) compared with Group 2 (23.60 ± 5.63) (p=0.001). Mean tear film breakup time was lower in Group 1 (11.78 ± 4.07) compared with Group 2 (15.1 ± 1.6) (p=0.013). Moreover, mean meibomian gland area loss was 25.01% ± 10.04% in Group 1 and 16.54% ± 6.02% in Group 2 (p=0.002). Conjunctival cytologic analysis in Group 1 revealed grade 0 changes in 6 patients (27.3%), grade 1 changes in 14 patients (63.6%), and grade 2 changes in 2 patients (9.1%), whereas 18 patients (90%) had grade 0 changes and 2 patients (10%) had grade 1 changes (p=0.001) in Group 2.
Conclusions: The study demonstrates several ocular surface changes in children with Hashimoto’s thyroiditis. These findings may indicate a tendency for dry eye in pediatric Hashimoto’s thyroiditis patients without clinical evidence of thyroid-associated ophthalmopathy.
Keywords: Dry eye syndrome; Hashimoto’s disease; Tears; Osmolar concentration; Ocular surface; Children
RESUMO
Objetivo: Avaliar as características da superfície ocular em crianças com tireoidite de Hashimoto sem oftalmopatia associada à tireoide e comparar os resultados com aqueles de crianças saudáveis.
Métodos: Vinte e duas crianças com tireoidite de Hashimoto (Grupo 1) e 20 crianças saudáveis sem qualquer distúrbio ocular e/ou sistêmico (Grupo 2) participaram do estudo. Utilizou-se o questionário Índice da Doença da Superfície Ocular, medida de osmolaridade do filme lacrimal (Tearlab Osmolarity System, San Diego, CA, EUA), teste de Schirmer e tempo de ruptura do filme lacrimal, meibografia e citologia do raspado conjuntival e comparação dos resultados entre os grupos.
Resultados: O grupo de estudo incluiu 19 meninas e 3 meninos no Grupo 1 e 12 meninas e 8 meninos no Grupo 2 (p=0,081). A oftalmopatia associada à tireoide não foi identificada em nenhum dos pacientes. A média da osmolaridade do filme lacrimal foi 310,23 ± 11,98 mOsm/l no Grupo 1 e 313,60 ± 15,03 mOsm/l no Grupo 2 (p=0,424). A média do escore do teste de Schirmer foi menor no Grupo 1 (14,91 ± 6,27) do que no Grupo 2 (23,60 ± 5,63) (p=0,001). A média do tempo de ruptura do filme lacrimal foi menor no Grupo 1 (11,78 ± 4,07) em comparação com o Grupo 2 (15,1 ± 1,6) (p=0,013). Além disso, a média da perda de área da glândula meibomiana foi 25,01% ± 10,04% no Grupo 1 e 16,54% ± 6,02% no Grupo 2 (p=0,002). A análise da citologia conjuntival no Grupo 1 revelou alterações de grau 0 em 6 pacientes (27,3%), alterações de grau 1 em 14 pacientes (63,6%) e alterações de grau 2 em 2 pacientes (9,1%), enquanto 18 pacientes (90%) com alteração de grau 0 e 2 pacientes (10%) com alteração de grau 1 (p=0,001) no Grupo 2.
Conclusões: O estudo demonstra várias alterações da superfície ocular em crianças com tireoidite de Hashimoto. Esses achados podem indicar uma tendência para olho seco em pacientes pediátricos com tireoidite de Hashimoto, sem evidências clínicas de oftalmopatia associada à tireoide.
Descritores: Síndrome do olho seco; Doença de Hashimoto; Lágrimas; Concentração osmolar; Superfície ocular; Crianças
INTRODUCTION
Hashimoto’s thyroiditis (HT) is a chronic inflammation of the thyroid gland. It is considered the most common autoimmune disease and the most common cause of hypothyroidism(1). HT is an organ-specific autoimmune disorder characterized by diffuse goiter with lymphocytic infiltration(2). The principal biochemical characteristic of HT is the presence of thyroid autoantibodies against thyroid peroxidase (TPO) and thyroglobulin (TG), which are the two major thyroid antigens(3). Affected children may be asymptomatic, and thyroid function may range from euthyroidism to overt hypothyroidism or rarely hyperthyroidism(4).
Thyroid-associated ophthalmopathy (TAO) is a chronic autoimmune disorder typically associated with Graves’ disease and occasionally with HT. The clinical presentation of TAO includes proptosis, lid retraction, lid lag, restrictive extraocular myopathy, optic neuropathy, and inflammatory changes on the ocular surface(5). Ocular findings of TAO consist of exophthalmos, widening of palpebral fissure, lagophthalmos, and reduced tear production. These changes are responsible for the development of dry eye(6,7).
Little is known about ocular surface alterations in pediatric HT patients. One study demonstrated a preponderance of dry eye in adult HT patients as compared with controls in the presence of proptosis and low thyroid hormone levels. However, the study findings were solely based on Schirmer and tear film breakup time (TFBUT) test results(8).
In the present study, we aimed to investigate the ocular surface characteristics in a pediatric cohort with HT in the absence of TAO and compare the results with those of healthy subjects. We performed a panel of analyses including the Ocular Surface Disease Index (OSDI) questionnaire, tear film osmolarity (TFO) measurement, Schirmer and TFBUT tests, meibography, and conjunctival cytology assessment.
METHODS
A total of 42 children were enrolled in this prospective study following approval by the institutional review board. The study was performed between January 2015 and May 2015 in the Ophthalmology Clinic of Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital. The study followed the principles of the Declaration of Helsinki. The study included two groups: Group 1 comprised children with HT and Group 2 was an age-matched control group including healthy children without any ocular and/or systemic disorders. The parents of all patients gave informed consent before the procedures.
The diagnosis of HT is based on clinical, laboratory, and radiologic findings. For a definitive diagnosis of HT, seropositivity for TG autoantibodies and/or TPO autoantibodies, accompanied by at least one feature is required. These features are enlarged thyroid gland, abnormal thyroid function, and morphological changes on thyroid ultrasound(9). All participants were aged ≤17 years and required to be euthyroid (normal TSH and free T4 levels with or without treatment). Ophthalmological investigation showed that none of the participants had clinical evidence of TAO. In all patients, TSH and free T4 levels were within normal ranges before the assessment. Patients with additional systemic chronic disease or those receiving any other medication were excluded from the study.
All children underwent a routine ocular examination including best-corrected visual acuity, slit lamp biomicroscopic examination, fundoscopic evaluation, and intraocular pressure (IOP) measurement. Children with any other ocular disorder were excluded. The following tests were performed in each subject in the same order to determine the ocular surface characteristics: OSDI questionnaire, TFO measurement (TearLab Osmolarity System, TearLab Corp., San Diego, CA, USA), Schirmer test with anesthesia, TFBUT, meibography (Sirius Topography, CSO, Florence, Italy), and conjunctival brush cytology.
The OSDI questionnaire
The OSDI is a 12-item questionnaire that includes five different levels of symptoms related to dry eyes. Each patient scored the frequency of these symptoms on a 0 to 4 scale, where 0 = none of the time, 1 = some of the time, 2 = half of the time, 3 = most of the time, and 4 = all of the time. The total OSDI score was calculated on the basis of the following formula: OSDI = [(sum of scores for all questions answered) × 100]/[(total number of questions answered) × 4]. The OSDI has good sensitivity and specifity for distinguishing between normal subjects and those with dry eye disease(10).
Tear film osmolarity measurements
The TearLab Osmolarity System (TearLab Corp.) was used to measure TFO in all patients. Measurements were performed by the same investigator in the same examination room with a controlled temperature of 23.5°C to 25.9°C and humidity of 33%-40%. We obtained a single measurement from both eyes of the patients. Tear samples were collected by touching the tip of a test card to the surface of the lateral inferior tear meniscus of both eyes. A single-use disposable test chip mounted to a collection pen was used for measurements with the TearLab Osmometer. In total, 50 nL of tears were taken up into the microchannels of each test chip by passive capillary action. Subsequently, the pen was connected to the reader, and the result was displayed on a portable reader unit in milliosmoles per liter.
Schirmer and TFBUT tests
The Schirmer test was conducted under topical anesthesia to assess tear secretion in each eye of the patients and controls. A standard Schirmer test strip was placed in the lower fornix at the junction of the lateral and middle third, taking care to avoid the cornea. The strip was removed after 5 min, and the wetted length of the test strip was measured in millimeters to determine the Schirmer test value. TFBUT analysis was performed 3 min after topical anesthesia administration. After 3 min, a fluorescein strip was wetted by a drop of physiological saline and touched to the inferior fornix. The subjects were asked to blink 3 times. Next, they looked straight ahead without blinking. The precorneal tear film was examined under blue-light illumination, noting the first time break of this layer.
In addition, corneal and conjunctival fluorescein staining was evaluated using the Oxford scheme consistent with the literature(11).
Meibography analysis
The Sirius Scheimpflug rotating camera system (Sirius Topography, CSO, Florence, Italy), which has a built-in infrared camera, was used to image the meibomian gland. Images were taken from the upper and lower eyelids of both eyes. Digital analysis of gland dysfunction and the severity of meibomian gland area (MGA) loss were calculated automatically after visual documentation of the glands.
Conjunctival brush cytology assessment
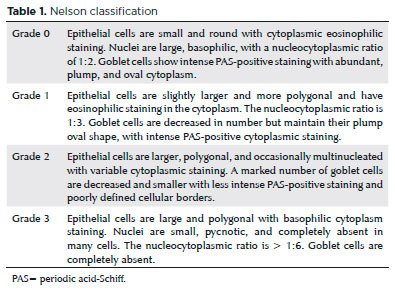
Conjunctival brush cytology specimens were acquired after administration of topical anesthesia with 0.5% proparacaine as described previously(12). The central upper bulbar conjunctiva was used for sampling. The conjunctiva was scraped by rotating the Cytobrush-S (Medscand AB, Malmö, Sweden) 360°. The brushes were fixed in PreservCyt solution, and the cells were detached from the brush by shaking the containers. The suspended cells were fixed in the ThinPrep processor according to the manufacturer’s directions, and ThinPrep slides were obtained at the end of the procedure. The slides were stained using the Papanicolaou method. Periodic acid-Schiff staining was performed to determine the goblet cells per slide, and the goblet cells were counted using 200× magnification. The Nelson method was used to evaluate conjunctival cytology (Table 1)(13). The same cytopathologist, who was unaware of the group assignment, performed all of the examinations.
The data were analyzed using SPSS Statistical Software (SPSS for Windows 11.0, Chicago, IL, USA). A p-value <0.05 was considered statistically significant.
RESULTS
In total, 22 patients (19 girls and 3 boys) comprised Group 1 and 20 healthy subjects (12 girls and 8 boys) made up Group 2. No statistically significant differences were observed between the groups with regard to sex, age, refraction status, or IOP (Table 2). The mean duration of HT was 20.36 ± 17.95 months (range, 2-84 months). Anti-TG antibodies were present in 90.9%, and anti-TPO antibodies were found in 81.8%. The mean TSH level was 2.95 ± 1.29 uIU/mL (range, 1.07-6 uIU/mL) with a reference range of 0.7-6.4 uIU/mL, and the mean free T4 level was 1.08 ± 0.15 ng/dL (range, 0.8-1.49 ng/dL) with a reference range of 0.8-2.2 ng/dL. The disease was controlled in all patients in Group 1, and no patient was on immunosuppression therapy.
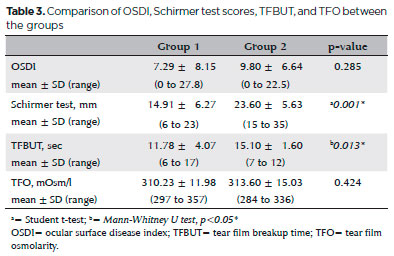
Ocular surface staining with fluorescein was not performed in either group. There was no ocular surface staining with fluorescein in both groups. Results of the OSDI, Schirmer test, TFBUT analysis, and TFO measurements are presented in table 3. The Schirmer and TFBUT test results were significantly lower in Group 1 compared with Group 2 (p=0.001 and p=0.013, respectively). No significant differences were observed in the OSDI (p=0.285) or TFO measurements (p=0.424).
Morphological evaluation of the meibomian gland showed 25.01% ± 10.04% (range, 9.9%-50.4%) of MGA loss in Group 1 and 16.54% ± 6.02% (range, 8%-28.9%) of MGA loss in Group 2 (p=0.002).
Conjunctival cytology analysis of Group 1 revealed grade 0 changes in 6 patients (27.3%), grade 1 changes in 14 patients (63.6%), and grade 2 changes in 2 patients (9.1%) in Group 1, whereas Grade 2 had 18 patients (90%) with grade 0 changes and 2 patients (10%) with grade 1 changes (p=0.001).
DISCUSSION
Several studies have reported that the presence of TAO predisposes patients to ocular surface changes leading to dry eye(14-17). Exophthalmos, a well-known condition in Graves’ ophthalmopathy, is an important cause of dry eye. However, some newly diagnosed Graves’ disease patients with no apparent TAO have demonstrated ocular surface damage(18). No patient had any evidence of TAO in our study. In addition, TSH and free T4 levels were within normal ranges, indicating that HT was controlled in the patient group. Nevertheless, our study results indicated a preponderance of dry eye in children with HT, even in the absence of TAO-related findings and abnormal hormone levels.
Each patient in the present study was required to answer the OSDI Questionnaire since subjective complaints are also clinically important. The scores were expected to correlate with the degree of ocular surface changes; however, we found no difference in OSDI scores between the study and control groups. One study reported OSDI scores implying that children with mild ocular surface damage report fewer dry eye symptoms compared with adults(19). Assessment of OSDI may have limited benefit for detecting dry eye in children due to poor compliance and difficulty understanding the instructions(20,21).
Increased TFO levels might be relevant in the development of dry eye in patients with thyroid eye disease(22). TFO has been suggested to induce inflammation leading to loss of epithelial and goblet cells(23). The mean TFO of children with HT was similar to that of healthy children in our study. Similar TFO levels between the study groups is possibly due to the absence of TAO-related findings. However, TFO levels can reveal alterations as ophthalmopathic status worsens during clinical follow-up.
Kan et al.(8) found reduced Schirmer and TFBUT measurements with higher OSDI scores in adult HT patients and also identified an association of dry eye with proptosis and lower thyroid hormone levels. The authors proposed that increased palpebral width due to proptosis may expedite tear film evaporation causing tear film hyperosmolarity and dry eye. Similarly, the patient group had lower Schirmer and TFBUT results compared with the control group in our pediatric HT patients.
The present study has produced some novel findings that are reported for the first time in the literature. Conjunctival brush cytology showed significantly reduced goblet cell density in pediatric HT patients. Although brush cytology has been conventionally used to obtain cells from cervicovaginal smears(24), this method demonstrated advantages of easier sample acquisition, simple preperation, and good sample quality in children(25-27). Furthermore, children with HT demonstrated significant MGA loss on meibographic analysis. Meibography is a relatively recent method used to detect meibomian gland dysfunction(28). Studies have also revealed a relationship between tear film instability and MGA loss(29).
The main limitations of our study were the relatively small sample size and not evaluating the effect of pubertal status on meibomian gland function. The age- and sex-related difference in serum sex hormone levels may be responsible for the differences in meibomian gland secretions between boys and girls; however, the basis for these differences is not fully understood(30). Further evaluation is needed to understand the relationship between sex steroids and ocular surface alterations.
In conclusion, we demonstrated several ocular surface changes in children with HT despite the shorter duration of the disease. Significantly lower Schirmer and TFBUT test results along with higher MGA loss and significant conjunctival cytologic changes were seen in the study group when compared with the controls. On the basis of these findings, we can assume that pediatric HT patients have a tendency for dry eye, even when thyroid hormone levels are normal without TAO. Pediatricians should always consider ocular surface manifestations in patients with HT. During follow-up, these changes might occur or worsen, and periodic investigation might be necessary. Further prospective large series studies are needed to validate the results of the present study.
REFERENCES
1. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13(4-5):391-7.
2. Hiromatsu Y, Satoh H, Amino N. Hashimoto’s thyroiditis: history and future outlook. Hormones (Athens). 2013;12(1):12-8.
3. Zaletel K, Gaberšček S. Hashimoto’s Thyroiditis: From Genes to the Disease. Curr Genomics. 2011;12(8):576-88.
4. De Luca F, Santucci S, Corica D, Pitrolo E, Romeo M, Aversa T. Hashimoto’s thyroiditis in childhood: presentation modes and evolution over time. Ital J Pediatr. 2013;39:8.
5. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2000;21(2): 168-99.
6. Eckstein AK, Finkenrath A, Heiligenhaus A, Renzing-Köhler K, Esser J, Krüger C, et al. Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand. 2004;82(3 Pt 1):291-7.
7. Gilbard JP, Farris RL. Ocular surface drying and tear film osmolarity in thyroid eye disease. Acta Ophthalmol (Copenh). 1983; 61(1):108-16.
8. Kan E, Kılıçkan E, Ecemiş G, Beyazyildiz E, Çolak R. Presence of Dry Eye in Patients with Hashimoto’s Thyroiditis. J Ophthalmol. 2014;2014:754923.
9. de Vries L, Bulvik S, Phillip M. Chronic autoimmune thyroiditis in children and adolescents: at presentation and during long-term follow-up. Arch Dis Child. 2009;94(1):33-7.
10. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615-21.
11. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22(7):640-50.
12. Wakamatsu TH, Okada N, Kojima T, Matsumoto Y, Ibrahim OM, Dogru M, et al. Evaluation of conjunctival inflammatory status by confocal scanning laser microscopy and conjunctival brush cytology in patients with atopic keratoconjunctivitis (AKC). Mol Vis. 2009;15:1611-9.
13. Nelson JD. Impression cytology. Cornea. 1988;7(1):71-81.
14. Gürdal C, Saraç O, Genç I, Kırımlıoğlu H, Takmaz T, Can I. Ocular surface and dry eye in Graves’ disease. Curr Eye Res. 2011;36(1):8-13.
15. Achtsidis V, Tentolouris N, Theodoropoulou S, Panagiotidis D, Vaikoussis E, Saldana M, et al. Dry eye in Graves ophthalmopathy: correlation with corneal hypoesthesia. Eur J Ophthalmol. 2013; 23(4):473-9
16. Selter JH, Gire AI, Sikder S. The relationship between Graves’ ophthalmopathy and dry eye syndrome. Clin Ophthalmol. 2014; 9:57-62.
17. Brasil MV, Brasil OF, Vieira RP, Vaisman M, Amaral Filho OM. [Tear film analysis and its relation with palpebral fissure height and exophthalmos in Graves’ ophthalmopathy]. Arq Bras Oftalmol. 2005;68(5):615-8.
18. Bruscolini A, Abbouda A, Locuratolo N, Restivo L, Trimboli P, Romanelli F. Dry Eye Syndrome in Non-Exophthalmic Graves’ Disease. Semin Ophthalmol. 2015;30(5-6):372-6.
19. Han SB, Yang HK, Hyon JY, Hwang JM. Children with dry eye type conditions may report less severe symptoms than adult patients. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):791-6.
20. Greiner KL, Walline JJ. Dry eye in pediatric contact lens wearers. Eye Contact Lens. 2010;36(6):352-5.
21. Alves M, Dias AC, Rocha EM. Dry eye in childhood: epidemiological and clinical aspects. Ocul Surf. 2008;6(1):44-51.
22. Iskeleli G, Karakoc Y, Abdula A. Tear film osmolarity in patients with thyroid ophthalmopathy. Jpn J Ophthalmol. 2008;52(4):323-6.
23. Bron AJ, de Paiva CS, Chauhan SK, Bonini S, Gabison EE, Jain S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017; 15(3):438-510.
24. Trimbos JB, Arentz NP. The efficiency of the Cytobrush versus the cotton swab in the collection of endocervical cells in cervical smears. Acta Cytol. 1986;30(3):261-3.
25. Tsubota K, Kajiwara K, Ugajin S, Hasegawa T. Conjunctival brush cytology. Acta Cytol. 1990;34(2):233-5.
26. Yağmur M, Ersöz C, Ersöz TR, Varinli S. Brush technique in ocular surface cytology. Diagn Cytopathol. 1997;17(2):88-91.
27. Wang Y, Dogru M, Matsumoto Y, Ward SK, Ayako I, Hu Y, et al. The impact of nasal conjunctivochalasis on tear functions and ocular surface findings. Am J Ophthalmol. 2007;144(6):930-7.
28. Finis D, Ackermann P, Pischel N, König C, Hayajneh J, Borrelli M, et al. Evaluation of Meibomian Gland Dysfunction and Local Distribution of Meibomian Gland Atrophy by Non-contact Infrared Meibography. Curr Eye Res. 2015;40(10):982-9.
29. Han KE, Yoon SC, Ahn JM, Nam SM, Stulting RD, Kim EK, et al. Evaluation of dry eye and meibomian gland dysfunction after cataract surgery. Am J Ophthalmol. 2014;157(6):1144-50.
30. Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014;97(4):324-36.
Approved by the following research ethics committee: Zeynep Kamil Maternity and Children’s Diseases Training and Research Hospital (#12.12.2014-20)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose