

Rosália M. S. Antunes-Foschini; Wanli Ho; André Messias
DOI: 10.5935/0004-2749.20180078
ABSTRACT
Purpose: To study visual acuity, refractive errors, eccentric fixation, and reading performance in patients with toxoplasmic macular retinochoroiditis.
Methods: Twenty-three participants with bilateral toxoplasmic macular retinochoroiditis and 4 with toxoplasmic macular retinochoroiditis in their unique eye were evaluated. Participants reported their eye dominance, confirmed by the Portus and Miles test. Best corrected visual acuity, spherical equivalent refraction, magnification need, and reading speed were measured. Microperimetry (MAIA, Centervue - Padova, Italy) recorded the preferred retinal locus and fixation stability by means of the bivariate contour ellipse area. Fourteen eyes from 14 normally sighted subjects served as controls.
Results: Mean ± SD best corrected visual acuity was better in the dominant eye than in the nondominant eye: 0.9 ± 0.2 (logMAR 0.5 to 1.4) vs. 1.2 ± 0.3 (logMAR 0.6 to 1.7) (p<0.0001, paired t-test). Spherical equivalent myopia of -4.00 or higher was present in 42% of the eyes. Microperimetry was performed in 42 eyes. Eccentric fixation was observed in all examined eyes. In 14 eyes (33%), the preferred retinal locus was placed (in the retina) superior temporal to the macular lesion, in 10 (24%) superior nasal, in 6 (14%) inferior temporal, and in 12 (28%) inferior nasal. There was no significant difference in the distribution of the preferred retinal locus position between dominant and nondominant eyes (p=0.85, Pearson test). There was no correlation between reading speed and the distance between the preferred retinal locus and the estimated original foveal position (r=-0.09; p=0.73), the bivariate contour ellipse area (r=-0.19; p=0.44), or best corrected visual acuity (r=0.024; p=0.92).
Conclusions: Myopia is more prevalent in patients with toxoplasmic macular retinochoroiditis. Reading speed is not dependent on preferred retinal locus position, stability, or visual acuity. Nevertheless, documentation of fixation provides new data on the impact of visual impairment in these patients and may be useful for rehabilitation efforts.
Keywords: Choroiditis; Chorioretinitis; Myopia; Toxoplasmosis, ocular; Reading
RESUMO
Objetivo: Estudar a acuidade visual, erros de refração, fixação excêntrica e desempenho de leitura em pacientes com retinocoroidite macular por Toxoplasmose.
Métodos: Vinte e três pacientes com retinocoroidite macular por Toxoplasmose bilateral e quatro com retinocoroidite macular por Toxoplasmose no seu único olho foram avaliados. Os participantes relataram sua dominância ocular, confirmada pelo teste de Portus e Miles. A acuidade visual melhor corrigida, refração em equivalente esférico, magnificação necessária e velocidade de leitura foram medidas. A microperimetria (MAIA, Centervue - Padova, Italy) registrou a estabilidade preferida do locus e da fixação da retina por meio da área da elipse de contorno bivariada. Quatorze olhos de 14 pacientes com boa visão serviram como controles.
Resultados: A média ± DP da acuidade visual melhor corrigida foi melhor no olho dominante do que no não dominante: 0,9 ± 0,2 (logMAR 0,5 a 1,4) vs. 1,2 ± 0,3 (logMAR 0,6 a 1,7) (p<0,0001, teste t pareado). Miopia em equivalente esférico de -4,00 ou maior estava presente em 42% dos olhos. Microperimetria foi realizada em 42 olhos. Fixação excêntrica foi observada em todos os olhos examinados. Em 14 olhos (33%), o locus retiniano preferencial estava localizado, na retina, na região súpero-temporal à lesão macular, em 10 (24%) súpero-nasal, em 6 (14%) ínfero-temporal, e em 12 olhos (29%) ínfero-nasal. Não houve diferença significativa na distribuição da posição do locus retiniano preferencial entre olhos dominantes e não-dominantes (p=0,85, teste de Pearson). Não houve correlação entre velocidade de leitura e distância entre o locus retiniano preferencial e a posição foveal original estimada (r=-0,09; p=0,73), a área bivariada de contorno elipsóide (r=-0,19; p=0,44) ou acuidade visual melhor corrigida (r=0,024; p=0,92).
Conclusões: A miopia é mais prevalente em pacientes com retinocoroidite macular por Toxoplasmose. A velocidade de leitura não é dependente da posição do locus retiniano preferencial, da estabilidade ou da acuidade visual. A documentação do padrão de fixação excêntrica, entretanto, oferece novos dados no impacto da deficiência visual nesses pacientes e pode ser útil em estratégias de reabilitação.
Descritores: Coroidite; Coriorretinite; Miopia; Toxoplasmose ocular; Leitura
INTRODUCTION
Toxoplasmosis is a disease caused by Toxoplasma gondii, an obligate intracellular parasite that causes zoonotic infection in humans and other mammals(1). Infection is most commonly acquired by ingesting undercooked meat containing the cystic bradyzoite form or ingesting material contaminated by cat feces, such as vegetables or water(2), that may contain oocysts. It affects people of all ages, and the infection is more common than the congenital form(3).
T. gondii infects up to a third of the world’s population and is responsible for the majority of cases of infectious intraocular uveitis(4). Unlike North America, where there are three predominant lineages, in South America there is greater genetic diversity, showing evidence of sexual recombination(5). There is a particular lineage of the parasite present in South America with a high incidence of ocular toxoplasmosis(3,6). Toxoplasmic macular retinochoroiditis (TMR) typically affects the posterior pole of the eyes(7), leading to vision impairment(8-10). Active lesions present as gray-white areas of retinal necrosis, and there may be other ocular complications, such as choroidal neovascularization, cataract, glaucoma, optic nerve atrophy, and retinal detachment(11,12). In Brazil, TMR is one of the leading causes of low vision in children(9,13).
The diagnosis of ocular toxoplasmosis is clinical in association with positive serology. There is no reliable diagnostic test to identify toxoplasmic uveitis. T. gondii IgG antibodies do not confirm the etiology, but a negative IgG generally eliminates the diagnosis.
Due to the macular scar, patients with bilateral toxoplasmic macular retinochoroiditis that occurred very early in life develop an adaptive strategy to use the peripheral retina in place of the damaged fovea. This location is known as the preferred retinal locus (PRL)(14,15).
The aim of this study was to report clinical findings, retinal position, and fixation patterns in patients with TMR and to compare them with eccentric fixations due to other causes.
METHODS
Ethical approval
The study protocol adhered to the tenets of the Declaration of Helsinki and was approved by the local Institutional Review Board. All participants gave written informed consent before entering the study.
Participants
Patients under 50 years of age with clinical TMR and IgG positive for toxoplasmosis were evaluated. All patients had both foveal regions affected by retinochoroiditis.
Measurements
Distant best corrected visual acuity (BCVA) was determined with the use of the Early Treatment Diabetic Retinopathy Study Chart (Lighthouse, Long Island, NY, USA)(16).
Near BCVA was determined with the use of phrases from the Colenbrander Low Vision Text Chart (Precision Vision, Woodstock, IL, USA). The participants were asked to choose the smallest letter size they could read at a comfortable distance, using their optical correction for distance. Near BCVA was calculated as the distance at which the text was read in meters divided by the size of the letter in M notation.
The magnification need to read text of 1M print size was calculated based on the patient’s near BCVA, using the Kestenbaum rule, as a starting value. The effective magnification need, defined as the number of additional positive lenses that allowed the patient to read 1M text with the greatest possible comfort, was calculated based on the new distance the patient chose to read 1M text, using positive lenses over the patient’s distance optical correction. For example, if an emmetropic patient was able to read 1M print at a distance of 0.05 meters, the patient’s magnification need was considered 20 D, whether or not he or she needed additional positive lenses or used by his accommodation effort or adding positive lenses.
Static refractometry was performed with retinoscopy using cyclopentolate, and the results were recorded as spherical equivalents. Indirect fundoscopy was also performed. Microperimetry was used to determine the PRL and estimate fixation stability by means of the bivariate contour ellipse area (BCEA)(17). The BCEA was automatically calculated based on 63% of the fixation points. In the case of more than one fixation locus, the most used PRL was considered.
Text reading speed with the dominant eye was measured using a standardized text, recording the number of words read per minute (wpm)(18) and the number of reading errors per text(19). One of 10 possible texts was chosen at random, and the patient was instructed to read the text as quickly as possible during a period ranging from 2 to 3 minutes. Reading speed was calculated as the number of words read minus the number of mistakes divided by the time in minutes. The original fovea position was estimated based on the optic nerve position according to a previously described method(20). The number of mistakes was standardized as the number of words read wrongly for every 100 words read.
The area of the macular scar was calculated based on optic nerve area measurement, using Image J (https://imagej.nih.gov/ij/), according to the method of Jonas et al.(21). The areas of the optic nerve and the macular scar were measured, and the macular scar area was determined based on the optic nerve area.
Statistical analysis
The D’Agostino & Pearson normality test was used to search for normality data. Nonparametric data were expressed as medians and ranges and parametric data as means ± standard deviation (SD). P values <0.05 were considered to indicate statistical significance. Student’s t-tests were used for parametric data, and the Mann-Whitney and Wilcoxon tests were used for nonparametric data. All analyses were performed using GraphPad Prism, version 7.0 (San Diego, CA, USA).
RESULTS
A total of 23 participants with bilateral TMR and 4 with TMR in the unique eye were evaluated; there were 50 eyes, all with the foveal region affected by retinochoroiditis. The male:female ratio was 17:10, with a mean ± SD age of 21.4 ± 11.0 years. The macular scars were round or oval-shaped and had a mean ± SD area of 14.1 ± 2.0 mm2 (n=16) in the dominant eyes and 11.5 ± 1.2 mm2 (n=12) in the nondominant eyes (p>0.29). The scar areas of the remaining participants could not be measured because the limits of the scar were not clear or the whole optic nerve head did not appear on microperimetry. Nystagmus was observed in 16 dominant eyes, 18 nondominant eyes, and 2 unique eyes.
Refractive spherical equivalents, BCVA, and magnification need
The refractive spherical equivalents of dominant eyes (n=27, median -2.50, ranging from -15.50 to + 4.00) and nondominant eyes (n=23, median -2.25, ranging from -15.5 to + 3.25) were not different (p=0.99, nonparametric unpaired t-test), with 42% of the eyes showing -4.00 D or higher spherical equivalent myopia.
Considering participants with bilateral TMR, the mean ± SD BCVA was better in the dominant eyes (logMAR 0.9 ± 0.2, ranging from 0.5 to 1.4) than in the nondominant eyes (logMAR 1.2 ± 0.3, ranging from 0.6 to 1.7) (n=23; p<0.0001, paired t-test). The participants with an unique eye had a BCVA of logMAR 1.0 (n=2) and logMAR 1.1 (n=2).
The effective magnification need to read 1M print was + 8.00 ± 3.00 D, ranging from + 4.00 to + 16.00 D.
Microperimetry
Microperimetry was performed in 42 eyes of 25 participants: in both eyes of 17 participants, in 3 unique eyes of 3 participants, and in 5 eyes of the other 5 participants. In five eyes, the examination was aborted due to poor visualization of the fixation target, a red circle of about 0.5 mm. Due to the presence of macular scars, eccentric fixation was observed in all 42 eyes. BCEA 63% was significantly different between dominant and nondominant eyes (n=17) (median of differences, 1.7; p=0.02, Wilcoxon matched-pairs signed rank test).
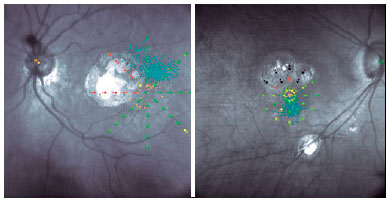
In 14 eyes (33%) the PRL was located on the retina superior temporal (Figure 2 left shows one example) to the macular lesion, in 10 (24%) eyes superior nasal, in 6 eyes (14%) inferior temporal, and in 12 eyes (29%) inferior nasal (Figure 2 right shows one example). There was no significant difference between dominant and nondominant eyes in the distribution of PRL position (p>0.05, paired t-test).
Reading speed
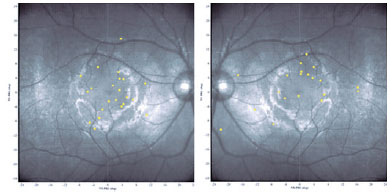
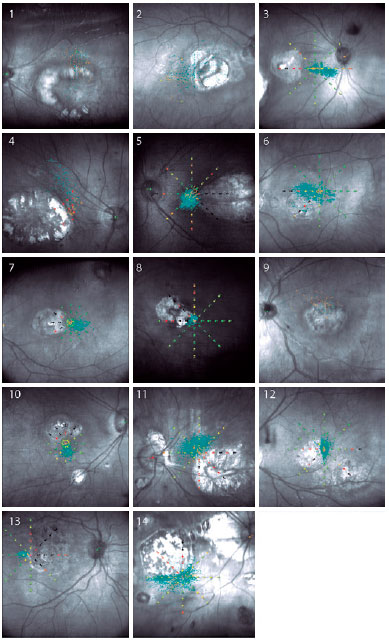
The reading speed of 17 participants (in the dominant eyes) and 3 participants with a unique eye (n=20 participants) was recorded. Measurements were not performed on the nondominant eyes due to difficulty in reading mentioned by the participants, even when the BCVA from both eyes was similar. The mean ± SD BCVA in this subgroup was logMAR 0.9 ± 0.2 (ranging from 0.5 to 1.4). The median text reading speed was 54.2 wpm (reference, 180.2 wpm), ranging from 32.9 to 140. The mean ± SD number of mistakes per 100 words read was 3.0 ± 3.0 (ranging from 0 to 10.1). There was no significant correlation between reading speed and number of mistakes (n=20 participants) (r=0.43; p=0.053). The fixation patterns of the dominant eyes of 14 participants are shown in figure 1.
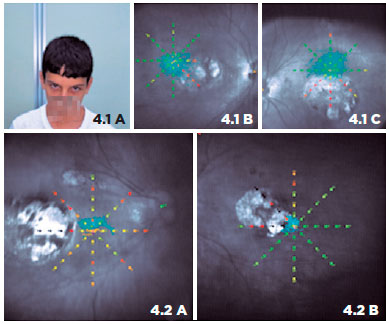
Figure 4 shows the association between PRL and the abnormal head position adopted by some patients during activities of daily living. Patient 1 has a chin-down head position, and microperimetry shows PRL located superiorly (left eye dominant). Patient 2 has the face turned to the right, maintaining the eyes in levoversion. The right eye shows a PRL nasal to the macular scar, and the left eye shows a PRL temporal to the lesion.
In the dominant eyes of 17 participants and unique eye of 3 participants, there were no significant correlations between reading speed and distance of the estimated original foveal position and PRL (2 participants did not undergo microperimetry (n=18 participants) (r=-0.09; p=0.73), or BCEA (2 participants did not undergo microperimetry) (n=18 participants) (r=-0.03; p=0.89) or BCVA (n=20 participants) (r=-0.12; p=0.60), indicating that the reading speed is not dependent on PRL position, fixation stability, or visual acuity. There were also no significant correlations between scar area in the dominant eyes and BCVA (n=16 participants) (r=0.31; p=0.23) or spherical equivalent (n=16 participants) (r=-0.17; p=0.52).
DISCUSSION
While only a small percentage of patients with toxoplasmosis retinochoroiditis have both macular areas affected(22), bilateral TMC remains one of the most prevalent causes of low vision in Brazil in young people from an early age(13,23). We examined a group of patients with macular scars due to toxoplasmosis, which is assisted by our Low Vision Service. Although it is not possible to identify the precise route of transmission (vertical during pregnancy or horizontal after birth through the ingestion of contaminated water or food), all patients had a history of low vision, many with nystagmus or strabismus, from an early age. They also had typical toxoplasmic macular scars, were IgG positive for toxoplasmosis, and showed no evidence of other infectious diseases that could lead to retinochoroiditis.
According to the 10th Revised International Classification of Diseases and Health-Related Problems, 3 patients (11%) had mild visual impairment (logMAR 0.5), 11 (41%) had low vision grade category 1 (logMAR 0.6 to 0.9), 12 (44%) had low vision grade category 2 (logMAR 1.0 to 1.3), and 1 (4%) had low vision grade category 3 (logMAR 1.4)(24).
With regard to refractive errors, Brazilian studies have shown a prevalence of myopia ranging from 2.8% to 3.8% in children up to 10 years of age(25,26) about 5% to 6% at ages 11 to 14 years(27), 19.3% at ages 16 to 18 years(26) 13.3% at ages 5 to 46 years(28) and 29.7% in a population aged 30 to 39 years(25). In our study, 66.6% (18 of 27) of the participants had myopia in both eyes, and 42% (21 of 50) of the eyes had myopia of -4.00 spherical D or more. A possible cause of myopia in these patients may be the presence of bilateral macular scars. Although the peripheral retina seems to regulate emmetropizing responses more than the central retina(29), the higher frequency of myopia might be explained by form-deprivation myopia, that is, the obscured images due to macular scars from an early age would lead to secondary ocular growth(30).
Although myopia is a burden for the general population(31), the association of myopia and low vision ends up being beneficial to these low-vision patients, because it allows reading without the accommodative effort being too high or without the need for the use of positive lenses all the time. The amount of accommodation effort or the number of additional positive lenses the patients needed to read was approximately equal to 1/BCVA. As shown, the mean BCVA was logMAR 0.9 ± 0.2, which corresponds to a BCVA, in decimal notation, between 0.125 and 0.08, and their magnification need was + 8.5 ± 3.0 D. Frequently the number of additional positive lenses needed to read 1M print size text is greater than that estimated by Kestenbaum’s rule, as visual acuity reserve is important for comfortable reading(32). Most of our patients, however, are young and myopic, two conditions that are favorable for better functioning at small distances.
Participants were not given any formal training in fixation behavior, and we did not observe multiple PRLs. Because of the limitations of the microperimetry, we are not able to determine whether they used a unique PRL for activities of daily living, as well as for reading. However, observations of the positions of some patients’ eyes and their abnormal head positions during medical assistance are consistent with the PRLs found on microperimetry and can give us a clue that some, in fact, use the same PRL, as shown in figure 4. For example, figure 4.1.A shows a patient with an abnormal chin-down position, with eyes turned upwards, that is consistent with superior fixation (Figures 4.1.B and 4.1.C) with the dominant left eye. Moreover, Figures 4.2.A and 4.2.B show a PRL located temporal to the macular scar in the left eye and nasal to the macular scar in the right eye; this is in accordance with the abnormal head position turned to the right adopted by the patient (both eyes in levoversion).
Whereas reading speed (wpm) in our study was 54.2 wpm, Verdina et al.(33) found a mean reading speed of 67 wpm before rehabilitation training in patients with Stargardt disease. One possible explanation may be related to the size of their true scotomas represented by macular scars with a mean area of 14.1 ± 2.0 mm2 on their dominant eyes. As shown by Altpeter et al.(34) reading speed and scotoma size are negatively correlated. Second, socioeconomic conditions in which people are not accustomed to reading may explain slower reading speeds. Training and cognitive abilities may be substantial issues involved in the differences between reading speeds. Third, the mean ± BCVA was logMAR 0.9 ± 0.2 (ranging from 0.5 to 1.4), and in 13 of 27 patients it ranged from logMAR 1.0 to 1.4 in the better eye.
It is noteworthy that a larger macular scar area was observed in dominant eyes than in nondominant eyes. Although this difference was not statistically significant, it reinforces the importance of rehabilitation and close monitoring from childhood and of separate stimulation of the two eyes, since we do not know which eye will be the better one when the visual system is mature. If macular scars save peripheral foveal regions, we might consider that preventing amblyopia might provide better eccentric BCVA in both eyes.
We did not find correlations between reading speed and BCVA or PRL location. This finding agrees with that of other authors(35,36) who studied patients with macular degeneration. Fletcher et al.35 showed that reading speed decreased with decreasing visual acuity, but they found no correlation with PRL, as is shown in our data. Calabrèse et al.(37), however, showed a decrease in maximum reading speed associated with an increase in the distance between the PRL and the fovea.
Our study did not find a relationship between reading speed and fixation stability. This finding does not correspond with data from patients with acquired macular diseases, in whom a relationship exists between reading speed and fixation stability(38). Authors have even estimated that changes in fixation stability account for 54% of the variance in changes in reading speed and fixation stability in patients with newly developed macular disease. One possible explanation for the absence of correlation between reading speed and fixation stability is that patients with toxoplasmic macular lesions use different retinal areas to read and to fixate the target in microperimetry.
The precise quantification of fixation pattern by microperimetry provides new data about the impact of visual impairment in patients with TMR. Although our results indicate that there is no typical pattern of PRL placement, they might be useful for establishing rehabilitation strategies. Future work is needed to investigate whether these patients can be trained to improve their reading performance, as has already been demonstrated in patients with Stargardt disease, using a biofeedback microperimetric strategy(33).
REFERENCES
1. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000;30(12-13):1217-58.
2. Furtado JM, Winthrop KL, Butler NJ, Smith JR. Ocular toxoplasmosis I: parasitology, epidemiology and public health. Clin Experiment Ophthalmol. 2013;41(1):82-94.
3. Jones LA, Alexander J, Roberts CW. Ocular toxoplasmosis: in the storm of the eye. Parasite Immunol. 2006;28(12):635-42.
4. Commodaro AG, Belfort RN, Rizzo LV, Muccioli C, Silveira C, Burnier MN Jr et al. Ocular toxoplasmosis: an update and review of the literature. Mem Inst Oswaldo Cruz. 2009;104(2):345-50.
5. Su C, Khan A, Zhou P, Majumdar D, Ajzenberg D, Dardé ML et al. Globally diverse Toxoplasma gondii isolates comprise six major clades originating from a small number of distinct ancestral lineages. Proc Natl Acad Sci USA. 2012;109(15):5844-9.
6. Glasner PD, Silveira C, Kruszon-Moran D, Martins MC, Burnier Júnior M, Silveira S et al. An unusually high prevalence of ocular toxoplasmosis in southern Brazil. Am J Ophthalmol. 1992;114(2): 136-44.
7. Butler NJ, Furtado JM, Winthrop KL, Smith JR. Ocular toxoplasmosis II: clinical features, pathology and management. Clin Experiment Ophthalmol. 2013;41(1):95-108.
8. Lucena Dda R, Ribeiro JA, Lucena Dda R, de Lucena AL, Jorge R. [Retinal tears in toxoplasmic retinochoroiditis: case series]. Arq Bras Oftalmol. 2009;72(6):829-31. Portuguese.
9. Haddad MA, Lobato FJ, Sampaio MW, Kara-José N. Pediatric and adolescent population with visual impairment: study of 385 cases. Clinics (Sao Paulo). 2006;61(3):239-46.
10. Mets MB, Holfels E, Boyer KM, Swisher CN, Roizen N, Stein L, et al. Eye manifestations of congenital toxoplasmosis. Am J Ophthalmol. 1997;123(1):1-16.
11. Bosch-Driessen LH, Karimi S, Stilma JS, Rothova A. Retinal detachment in ocular toxoplasmosis. Ophthalmology. 2000;107(1):36-40.
12. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. 2005 Jul-Sep; 20(3):129-41.
13. Haddad MA, Sei M, Sampaio MW, Kara-José N. Causes of visual impairment in children: a study of 3,210 cases. J Pediatr Ophthalmol Strabismus. 2007;44(4):232-40.
14. Von Noorden GK, MacKensen G. Phenomenology of eccentric fixation. Am J Ophthalmol. 1962;53(4):642-60.
15. Whittaker SG, Budd J, Cummings RW. Eccentric fixation with macular scotoma. Invest Ophthalmol Vis Sci. 1988;29(2):268-78.
16. Bailey IL, Lovie-Kitchin JE. Visual acuity testing. From the laboratory to the clinic. Vision Res. 2013;90:2-9.
17. Reinhard J, Messias A, Dietz K, Mackeben M, Lakmann R, Scholl HP et al. Quantifying fixation in patients with Stargardt disease. Vision Res. 2007;47(15):2076-85.
18. Messias A, Velasco e Cruz AA, Schallenmüller SJ, Trauzettel-Klosinski S. [New standardized texts in Brazilian Portuguese to assess reading speed-comparison with four European languages]. Arq Bras Oftalmol. 2008;71(4):553-8. Portuguese.
19. Brussee T, van Nispen RM, Klerkx EM, Knol DL, van Rens GH. Comparison of reading performance tests concerning difficulty of sentences and paragraphs and their reliability. Ophthalmic Physiol Opt. 2015;35(3):324-35.
20. Rohrschneider K. Determination of the location of the fovea on the fundus. Invest Ophthalmol Vis Sci. 2004;45(9):3257-8.
21. Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988;29(7):1151-8.
22. Aleixo AL, Curi AL, Benchimol EI, Amendoeira MR. Toxoplasmic retinochoroiditis: clinical characteristics and visual outcome in a prospective study. PLoS Negl Trop Dis. 2016;10(5):e0004685.
23. de Paula CH, Vasconcelos GC, Nehemy MB, Granet D. Causes of visual impairment in children seen at a university-based hospital low vision service in Brazil. J AAPOS. 2015;19(3):252-6.
24. Organização Mundial da Saúde. Classificação Internacional de Doenças e Problemas Relacionados à Saúde - Décima Revisão. São Paulo: Edusp; 1993.
25. Schellini SA, Durkin SR, Hoyama E, Hirai F, Cordeiro R, Casson RJ et al. Prevalence of refractive errors in a Brazilian population: the Botucatu eye study. Ophthalmic Epidemiol. 2009;16(2):90-7.
26. Lira RP, Santo IF, Astur GL, Maziero D, Passos TH, Arieta CE. Refractive error in school children in Campinas, Brazil. Arq Bras Oftalmol. 2014;77(3):203-4.
27. Salomão SR, Cinoto RW, Berezovsky A, Mendieta L, Nakanami CR, Lipener C et al. Prevalence and causes of visual impairment in low-middle income school children in Sao Paulo, Brazil. Invest Ophthalmol Vis Sci. 2008;49(10):4308-13.
28. 28. Garcia CA, Oréfice F, Nobre GF, Souza DB, Rocha ML, Vianna RN. [Prevalence of refractive errors in students in Northeastern Brazil]. Arq Bras Oftalmol. 2005;68(3):321-5.
29. Smith EL 3rd, Ramamirtham R, Qiao-Grider Y, Hung LF, Huang J, Kee CS et al. Effects of foveal ablation on emmetropization and form-deprivation myopia. Invest Ophthalmol Vis Sci. 2007;48(9): 3914-22.
30. Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43(4):447-68.
31. Lou L, Yao C, Jin Y, Perez V, Ye J. Global Patterns in Health Burden of Uncorrected Refractive Error. Invest Ophthalmol Vis Sci. 2016; 57(14):6271-7.
32. Cheong AC, Lovie-Kitchin JE, Bowers AR. Determining magnification for reading with low vision. Clin Exp Optom. 2002;85(4):229-37.
33. Verdina T, Giacomelli G, Sodi A, Pennino M, Paggini C, Murro V et al. Biofeedback rehabilitation of eccentric fixation in patients with Stargardt disease. Eur J Ophthalmol. 2013;23(5):723-31.
34. Altpeter EK, Blanke BR, Leo-Kottler B, Nguyen XN, Trauzettel-Klosinski S. Evaluation of fixation pattern and reading ability in patients with Leber hereditary optic neuropathy. J Neuroophthalmol. 2013;33(4): 344-8.
35. Fletcher DC, Schuchard RA, Watson G. Relative locations of macular scotomas near the PRL: effect on low vision reading. J Rehabil Res Dev. 1999;36(4):356-64.
36. Crossland MD, Culham LE, Kabanarou SA, Rubin GS. Preferred retinal locus development in patients with macular disease. Ophthalmology. 2005;112(9):1579-85.
37. Calabrèse A, Bernard JB, Hoffart L, Faure G, Barouch F, Conrath J et al. Wet versus dry age-related macular degeneration in patients with central field loss: different effects on maximum reading speed. Invest Ophthalmol Vis Sci. 2011;52(5):2417-24.
38. Crossland MD, Culham LE, Rubin GS. Fixation stability and reading speed in patients with newly developed macular disease. Ophthalmic Physiol Opt. 2004;24(4):327-3.
Submitted for publication:
October 18, 2017.
Accepted for publication:
February 9, 2018.
Funding: This study was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 300749/2010-4), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2010/01714-9), and Fundação Apoio ao Ensino Pesquisa e Assistência (FAEPA) HCFMRP-USP
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Approved by the following research ethics committee: Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (# 1100/2010)