

Alicia Galindo-Ferreiro1; Rajiv Khandekar2; Sultan Al Hassan2; Fatimah Al-Hammad2; Hamad Al-Subaie2; Silvana Artioli Schellini2,3
DOI: 10.5935/0004-2749.20180073
ABSTRACT
Purpose: To evaluate the outcomes of dermis-fat graft procedure for orbital volume replacement in anophthalmic socket.
Methods: A retrospective chart review was performed studying all dermis-fat graft surgeries done at King Khlaed Eye Specialist Hospital in the last 10-year period. Sixty-two anophthalmic socket carriers underwent dermis-fat graft during the study period. Data were collected on characteristics of the patients, postoperative complications, cosmesis, and functional results such as the ability to hold an external prosthesis.
Results: Mean age of the patients included in the study was 34.2 ± 9.7 years. There were 38.7% of patients with grade 3 anophthalmic socket, 64.5% of patients had an acquired anophthalmic socket, and dermis-fat graft was performed as a secondary procedure in 61.3% of patients. Postoperative complications included lagophthalmos (22.6%), graft necrosis (17.7%), pyogenic granuloma (12.9%), decreased graft size (12.9%), malpositioned lids (3.2%), and volume deficiency (3.2%). The prosthesis was held in place in 49 patients (79%) preoperatively and in 55 patients (88.7%) postoperatively.
Conclusion: Dermis-fat graft is an excellent option for congenital or acquired as well as primary or secondary anophthalmic sockets, with or without contraction. The outcomes are favorable, and complications are rare.
Keywords: Anophthalmos/surgery; Orbit; Dermis; Adipose tissue/transplantation; Retrospective study
RESUMO
Objetivo: Avaliar os resultados obtidos com o uso do enxerto dermo-adiposo para reposição de volume em cavidade anoftálmica.
Métodos: Estudo retrospectivo baseado em revisão de prontuários incluindo todas as cirurgias de enxerto dermo-adiposo realizadas nos últimos 10 anos no King Khaled Eye Specialist Hospital, Saudi Arabia. O enxerto dermo-adiposo foi realizado em 62 pacientes no periodo do estudo. Os dados analisados incluíram características dos pacientes, as complicações pós- operatórias e os resultados cosméticos e funcionais, tais como a habilidade de usar prótese externa.
Resultados: A média de idade dos participantes foi de 34,2 ± 9,7 anos. Segundo a classificação das cavidades, 38,7% possuíam cavidade grau 3; 64,5% possuíam cavidade anoftálmica adquirida e o enxerto dermo-adiposo foi realizado como procedimento secundário em 61,3% dos pacientes. Após o procedimento 22,6% dos pacientes permaneceram com lagoftalmo, 17,7% tiveram necrose do enxerto, 12,9% desenvolveram granuloma piogênico, 12,9% tiveram redução do tamanho do enxerto, 3,2% permaneceram com as alterações no posicionamento palpebral e 3,2% continuaram com déficit de volume na órbita. Quarenta e nove pacientes (79%) eram capazes de usar prótese externa antes da cirurgia e depois do enxerto dermo-adiposo 55 (88,7%) puderam utilizar prótese externa.
Conclusão: O enxerto dermo-adiposo é uma ótima opção para tratamento de cavidades anoftálmicas congênitas ou adquiridas, assim como realizado como procedimento primário ou secundário, em cavidades com ou sem contração tecidual. Os resultados são encorajadores e as complicações são pouco frequentes.
Descritores: Anoftalmia/cirurgia; Órbita; Derme; Tecido adiposo/transplantes; Estudo retrospectivo
INTRODUCTION
The use of a dermis-fat graft (DFG) to replace volume in an anophthalmic cavity was first described in 1978(1,2). Enucleation or evisceration results in a significant decrease of orbital volume(3). DFG is currently considered an effective technique for replacing orbital volume in the socket(2) and may improve the anterior surface of the socket simultaneously. Significant volume may need to be replaced in cases of advanced phthisis bulbi, sockets that have undergone multiple surgeries, cases of implant extrusions associated with necrosis of conjunctiva and Tenon capsule. A DFG can be a good option for these cases.
DFG is an autologous implant that means there is no risk of transmission of infectious disease. Moreover, an autologous implant does not require special preparation, storage, or transportation, thereby reducing expenses. Lastly, this procedure can offer excellent cosmesis and functional results. These advantages have resulted in greater adoption of DFG recently(4).
However, some questions remain regarding the indications and outcomes of DFG. These questions spurred us to review the success rates and complications of DFG at our institute. To our knowledge, this is the largest sample size involving this relatively new procedure that has been reviewed.
METHODS
We performed a retrospective chart review of all primary and secondary DFG procedures carried out between March 2006 and December 2016 at the King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia. The study protocol was approved by the Ethics Committee of the Institution, and consent was waived due to the retrospective nature of the study. This study was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Patient confidentiality was maintained throughout the duration of this study.
Inclusion criteria were lack of volume in an anophthalmic cavity, sockets classified as grade 1-4 based on the contraction of the conjunctiva(5), with congenital or acquired anophthalmic socket, history of a primary or secondary DFG procedure, and participation in ≥6-month postoperative follow-up (Figure 1). Grade 5 sockets were excluded.
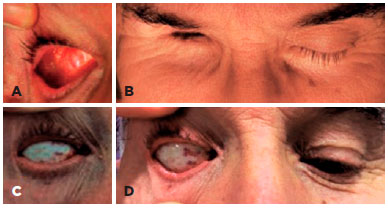
The DFG technique used was previously described(1,6). Briefly, a DFG harvested from the upper and lateral portion of the gluteus region and 30% larger than the orbital defect. The skin over the surface of the graft was excised, leaving only the graft composed by dermis and fat to be transposed to the anophthalmic socket. The graft was sutured to the Tenon capsule and conjunctiva using 4-0 and 6-0 polyglactin (Vicryl, Ethicon Inc., USA) interrupted sutures, respectively (Figure 2).
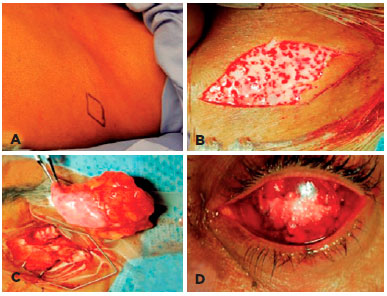
Primary DFG was defined as insertion of DFG immediately after enucleation or evisceration. Secondary DFG denoted history of undergoing surgery with implants in the socket, followed by implant removal and DFG. Lack of volume in the socket was defined as a deep superior sulcus and an enophthalmic cavity even with the external prosthesis in place.
Statistical analysis
Data were analyzed based on patient demographics, associated lid anomalies, conjunctival status, status of the fornix, subjective assessment of inadequate orbital volume, entropion/ectropion, lagophthalmos, scarred tissue in the socket, signs of infections, or other complications. The ability to hold a prosthesis in place was considered a successful outcome. A pretested collection form was used to collect data, which were then transferred to an Excel® spreadsheet (Microsoft Corp., Redmond, WA, USA). Statistical analysis was performed with Statistical Package for Social Sciences (SPSS 23; IBM Corp., New York, NY, USA). For quantitative variables with a normal distribution, the mean and standard deviation were calculated. For variables with a non-normal distribution, the median 25% quartile, minimum, and maximum values were calculated. For qualitative variables, the frequencies and percentage proportions were calculated. P<0.05 was considered statistically significant.
RESULTS
The study sample comprised 62 patients who underwent DFG. Patient demographics and ocular characteristics are presented in table 1. The mean age of the study sample was 34.2 ± 9.7 years (minimum 1.9 years and maximum 83.9 years). There were 36 females (58.1%). Twenty-four patients (38.7%) had a grade 3 anophthalmic socket. Forty patients (64.5%) underwent DFG to correct an acquired anophthalmic socket and 8 (12.9%) for congenital anophthalmia.
DFG was the primary surgery for 8 patients (12.9%); the remaining patients had undergone 1 (37.1%) to 5 (3.2%) previous procedures. Among patients who underwent DFG after other procedures, 35 (92%) underwent implant removal, and the DFG procedure was performed in the same surgical session. The implants were removed due to conjunctival and/or scleral dehiscence or lack of volume with the implant in place. These removed implants were composed of PMMA (19 patients; 54.3%), hydroxyapatite (7 patients; 20%), hydrogel (6 patients; 17.1%), bioceramic (2 patients; 5.7%), and silicone (1 patient; 2.9%).
Prior to the DFG procedure, 40 patients (66.7%) had inadequate orbital volume, 36 (58.1%) had a narrow and contracted fornix, 27 (43.5%) had a superior sulcus deformity, 22 (35.5%) had lagophthalmos with prosthesis, 3 (4.8%) had dehiscence with implant exposure, and 2 (3.2%) were diagnosed with entropion.
There were no intraoperative complications during the DFG surgical procedure. Postoperative findings/complications included lagophthalmos with the prosthesis in 14 patients (22.6%), partial necrosis of the DFG in 11 (17.7%), pyogenic granuloma in 8 (2.9%), decreased graft size in 8 (12.9%), volume deficiency remained in 4 (6.5%), and malpositioned lids (ptosis or entropion) in 2 (3.2%).
The prosthesis remained in place in 49 patients (79%) preoperatively and in 55 patients (88.7%) postoperatively.
DISCUSSION
There is increasing interest in DFG due to the easy accessibility of donor graft material, the low morbidity, and the minimal cost compared with the cost of alloplastic implants. Autologous graft eliminates the risk of rejection and foregoes access to a tissue bank or special handling and transportation procedures. Despite the need for second surgical field (donor site), it is commonly accepted that there is greater success in reconstructive procedures using autologous tissues compared with using donor tissue. For DFG, the donor site located at the lateral and superior quadrant of the gluteus region is easy to access, and the tissue can be removed quickly, causing mild postoperative pain and discomfort compared with other donor sites(7).
The majority of studies on DFG to date have enrolled small sample size, and there are no randomized studies(2,7-13). Hence, greater understanding is required for the indications and outcomes of this relatively new procedure.
Patient age is not a factor for DFG. In the current study, DFG was performed in patients ranging from 1.9 to 83.9 years old, and 18 patients had congenital anophthalmia/microphthalmia. DFG in infants allows slow and progressive orbital growth concurrent with the child’s growth, promoting orbital development and also creating an adequate fornix to maintain the external prosthesis, which is particularly advantageous in young patients(12-15). In the current study, lack of volume in the socket and shallow contracted fornix were the main indications for surgery. This observation concurs with a previous study that reported half of the patients undergoing DFG has shallow contracted fornix and volume insufficiency(7).
In the current study, DFG was used to treat patients with anophthalmic cavity grades 1-4 and 38.7% of our patients had grade 3 cavities. Although the DFG technique can be used in almost all kinds of sockets, cases with greater contraction benefit the most from this technique because the anterior surface of the socket is also replaced.
As reported by previous studies(10), DFG was a secondary procedure in the majority (87.1%) of patients in the current study. The most common clinical presentation prior to DFG in our study was an exposed or extruded implant. These observations concur with previous studies(2).
No intraoperative complication was observed in the current study, and major complications are rare with this technique(10,11). Additionally postoperative complications are seldom noted(2,4,7). Graft necrosis is one of the most severe adverse events after DFG and occurred in 17.7% of our patients similar to others(7) and higher than another report(8). Similar to a previous study(11), graft necrosis occurred more frequently after secondary DFG and was related to the technique to obtain the DFG or the implantation of the DFG in the orbit. Excessive manipulation or cauterization, multiple previous procedures, orbital radiotherapy, concomitant systemic vascular diseases (diabetes, hypertension, or others), immuno-suppression, or coagulation problems can be the causes to have necrosis of the graft. To enhance the success of the DFG, the rectus muscles must be meticulously sutured to the dermal edge of the graft, filleting Tenon’s capsule and avoiding cauterization of the graft bed(16).
We elected to harvest a DFG 30% larger than the measured orbital defect. The larger size is used to mitigate the potential for shrinkage and atrophy >40% after a secondary procedure(6) or 5%-10% after primary procedures(6). However, some surgeons have reported that the thicker the DFG, the better the outcome(17).
In the current study, graft atrophy with variable loss of volume occurred in 12.9% of the patients in the current study as observed by others(9), nevertheless other studies reported rates of 16.6% or 20%-40%(10). Differences in sample size and differing inclusion criteria may account for the varying rates of volume loss.
In the current study, lagophthalmos persisted in some patients likely due to inadequate fornix size and lid entropion.
Also we observed cysts over the DFG, which is a recognized complication, attributed to epithelial islands that remain on the DFG despite the preparation steps(8).
We had one patient with residual mild ptosis postoperatively, which also has been previously reported(8). Other reported postoperative complications of DFG include hair growth on the graft, wound dehiscence in the donor area(7), keratinization of the socket, graft wound dehiscence, donor wound hematomas(18), graft overgrowth requiring re-operation, or debulking(14). Some of these complications occur years after implantation and were not observed in the current study likely due to the relatively short follow-up period.
Postoperatively, 88.7% of our patients successfully held the external prosthesis, which is almost more than 10% preoperatively. Similar success rates have been previously reported(8,11).
The outcomes of the current study indicate that DFG can be used to reconstruct an anophthalmic socket as a primary or secondary implant, replacing volume and also improving the surface area of the socket, allowing greater adaptability to the external prosthesis. The increased volume in the socket is favorable for adaptation to a thinner external prosthesis, improving palpebral interaction and reducing the incidence of complications, resulting in better prosthesis mobility and palpebral stability(2-4,7).
The disadvantages of DFG include an increased risk of morbidity due to the necessity of a creating second surgical wound (donor site). Moreover, the DFG may shrink, and there is a lack of predictability(4). DFG surgery is more time consuming than socket reconstruction using alloplastic implants, but this technique overcomes some of the limitations of alloplastic implants such as dehiscence and/or extrusion(11).
The main limitation of our study is the retrospective nature and relatively short follow-up. However, the large sample size mitigates some of these limitations. The favorable outcomes in the current study should encourage others to perform a randomized controlled study with longer follow-up to assess the long-term effectiveness of DFG for anophthalmic cavity reconstruction.
In conclusion, DFG is a good option for congenital or acquired as well as primary or secondary anophthalmic socket reconstruction, with or without contraction. Complications such as graft necrosis or lid deformities are rare, and cosmesis and functional results were favorable.
REFERENCES
1. Smith B, Petrelli R. Dermis-fat graft as a movable implant within the muscle cone. Am J Ophthalmol. 1978;85(1):62-6.
2. Aryasit O, Preechawai P. Indications and results in anophthalmic socket reconstruction using dermis-fat graft. Clin Ophthalmol. 2015;9:795-9.
3. Vagefi MR, McMullan TF, Burroughs JR, et al. Autologous dermis graft at the time of evisceration or enucleation. Br J Ophthalmol. 2007;91(11):1528-31.
4. Al-Mujaini A, Ganesh A, Al-Zuhaibi S. Autogenous dermis-fat orbital impant for anophthalmic socket. Sultan Qaboos Univ Med J. 2007;7(2):145-8.
5. Tawfik HA, Zico OM. Orbital implants in postenucleation retinoblastoma. Ophthalmology. 2001;108(4):639-40.
6. Smith B, Bosniak S, Nesi F, Lisman R. Dermis-fat orbital implantation: 118 cases. Ophthalmic Surg. 1983;14(11):941-3.
7. Bengoa-Gonzalez A, Dolores Lago-Llinas M, Martin-Clavijo A, Ling-Tan S. The use of autologous dermis grafts for the reconstruction of the anophthalmic socket. Orbit. 2010;29(4):183-9.
8. Essuman VA, Tagoe NN, Ndanu TA, Ntim-Amponsah CT. Dermis-fat grafts and enucleation in Ghanaian children: 5 years’ experience. Ghana Med J. 2014;48(4):204-7.
9. Guberina C, Hornblass A, Meltzer MA, et al. Autogenous dermis-fat orbital implantation. Arch Ophthalmol. 1983;101(10):1586-90.
10. Kuzmanovic Elabjer B, Busic M, Bosnar D, et al. Our experience with dermofat graft in reconstruction of anophthalmic socket. Orbit. 2010;29(4):209-12.
11. Nentwich MM, Schebitz-Walter K, Hirneiss C, Hintschich C. Dermis fat grafts as primary and secondary orbital implants. Orbit. 2014; 33(1):33-8.
12. Quaranta-Leoni FM, Sposato S, Raglione P, Mastromarino A. Dermis-Fat Graft in Children as Primary and Secondary Orbital Implant. Ophthal Plast Reconstr Surg. 2016;32(3):214-9.
13. Tarantini A, Hintschich C. Primary dermis-fat grafting in children. Orbit. 2008;27(5):363-9.
14. Mitchell KT, Hollsten DA, White WL, O’Hara MA. The autogenous dermis-fat orbital implant in children. J AAPOS. 2001;5(6):367-9.
15. Heher KL, Katowitz JA, Low JE. Unilateral dermis-fat graft implantation in the pediatric orbit. Ophthal Plast Reconstr Surg. 1998; 14(2):81-8.
16. Bosniak SL. Dermis-fat orbital implantation and complex socket deformities. Adv Ophthalmic Plast Reconstr Surg. 1992;9:131-41.
17. Sihota R, Sujatha Y, Betharia SM. The fat pad in dermis fat grafts. Ophthalmology. 1994;101(2):231-4.
18. Bosniak SL. Avoiding complications following primary dermis-fat orbital implantation. Ophthal Plast Reconstr Surg. 1985;1(4):237-41. Submitted for publication: October 19, 2017 Accepted for publication: February 9, 2018
Submitted for publication:
October 19, 2017.
Accepted for publication:
February 9, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Approved by the following research ethics committee: King Khaled Eye Specialist Hospital (#1675-R)