

Bekir Küçük1; Serkan Akkaya2
DOI: 10.5935/0004-2749.20180072
ABSTRACT
Purpose: This study evaluated changes in choroidal and macular thickness in healthy volunteers and chronic smokers.
Methods: Thirty-three eyes of 33 chronic smokers (study group) and 33 eyes of 33 healthy controls who had never smoked were prospectively evaluated. Comprehensive ophthalmic assessment included slit lamp biomicroscopy, stereoscopic fundus examination, and intraocular pressure measurement. Spectral domain optical coherence tomography was used to measure choroidal and macular thickness 1 month before smoking cessation (smoking period) and after 3 months of smoking cessation (nonsmoking period).
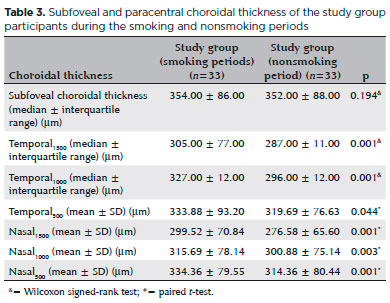
Results: The mean age of the participants was 41.88 ± 6.52 years (range, 26-52), and the average smoking duration was 8.6 ± 2.5 years (range, 5-16). The thickness of the paracentral choroid (nasal: 1,500 µm, p=0.001 and temporal: 1,500 µm, p=0.001) had significantly decreased after 3 months of smoking cessation. The thicknesses of the subfoveal choroid in the smoking and nonsmoking periods were not significantly different (p=0.194). The mean central macular thickness was 267.21 ± 18.42 µm in the smoking period and 268.42 ± 18.28 µm in the nonsmoking period (p=0.022).
Conclusions: Smoking was associated with statistically significant changes in paracentral choroidal and central macular thickness in healthy volunteers. Pathological studies should be performed to evaluate the effects of smoking on posterior ocular structures.
Keywords: Choroid/anatomy & histology; Macula lutea/anatomy & histology; Smoking cessation; Tomography, optical coherence
RESUMO
Objetivo: Este estudo avaliou as mudanças na espessura da coroide e da mácula em voluntários saudáveis e fumantes crônicos.
Métodos: Trinta e três olhos de 33 fumantes crônicos (grupo estudado) e 33 olhos de 33 controles saudáveis que nunca fumaram foram avaliados prospectivamente. A avaliação oftalmológica abrangente incluiu biomicroscopia de lâmpada de fenda, exame de fundo estereoscópico e medição da pressão intraocular. A tomografia de coerência óptica de domínio espectral foi utilizada para medir a espessura da coroide e da mácula um mês antes da cessação do tabagismo (período de fumar) e após 3 meses da cessação do tabagismo (período de abstinência).
Resultados: A idade média dos participantes foi de 41,88 ± 6,52 anos (faixa de 26-52 anos) e a duração média do tabagismo foi de 8,6 ± 2,5 anos (faixa, 5-16 anos). A espessura da coroide paracentral (nasal: 1.500 µm, p=0,001, temporal: 1.500 µm, p=0,001) diminuiu significativamente após 3 meses de cessação do tabagismo. As espessuras de coroide subfoveal nos períodos de tabagismo e não-tabagismo não foram significativamente diferentes (p=0,194). A espessura macular central média foi de 267,21 ± 18,42 µm no períodos de tabagismo e 268,42 ± 18,28 µm no períodos de não-fumantes (p=0,022).
Conclusões: O tabagismo foi associado a mudanças estatisticamente significativas na espessura paracentral de coroide e macular central em voluntários saudáveis. Estudos patológicos devem ser realizados para avaliar os efeitos do tabagismo nas estruturas oculares posteriores.
Descritores: Coroide/anatomia & histologia; Mácula lútea/anatomia & histologia; Abandono do hábito de fumar; Tomografia de coerência óptica
INTRODUCTION
Smoking is the leading preventable cause of death worldwide and the most damaging preventable lifestyle factor affecting public health globally and nationally(1). Smoking-related causes of death include lung cancer, chronic obstructive pulmonary disease, and cardiovascular diseases(2). Smoking is also associated with ocular vascular diseases such as hypertensive retinopathy, age-related macular degeneration, and anterior ischemic optic neuropathy(3).
Varenicline and bupropion are first-line medications indicated for smoking cessation. Varenicline was approved by the US Food and Drug Administration as a smoking cessation treatment(4,5). It is a high-affinity partial nicotinic agonist at the α2β4 nicotinic receptor and a full agonist at the neuronal α7 nicotinic receptor(6). Bupropion was initially approved for the treatment of major depressive disorders(7) and subsequently licensed as a smoking cessation agent. It is currently available in the United States as a first-line treatment to stop smoking(8). Bupropion acts by inhibiting dopamine and norepinephrine reuptake, and it weakens the stimulatory effects of nicotine on nicotinic acetylcholine receptors(9).
Age(10), axial length(11), chorioretinal diseases(12), severe myopia(13), and smoking(14) have been shown to affect choroid and macular thickness. The effects of smoking on the choroidal layer and ocular function have been studied(14-18), but data on the effects of smoking cessation on posterior ocular structures are lacking. Spectral domain optical coherence tomography (SD-OCT) is a noninvasive, noncontact, highly sensitive modality that provides high-resolution ophthalmic images and can be used to measure choroid layer thickness. This study was designed to objectively assess the effects of smoking cessation on the retina and choroid using SD-OCT.
METHODS
Study population and design
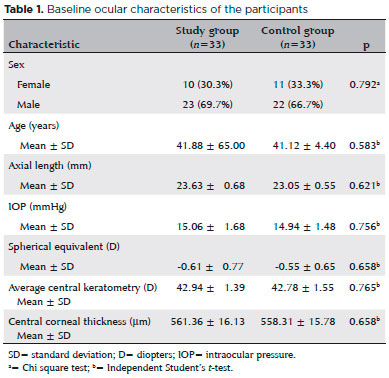
This prospective study was performed at the Department of Ophthalmology and Chest Diseases Hospital, Smoking Cessation Policlinic at, Kayseri Education and Research Hospital, Turkey. The study adhered to the tenets of the Declaration of Helsinki and was approved by the local ethics committee. The participants were given oral and written information about the study, and all provided written informed consent. Thirty-three eyes of 33 chronic smokers and 33 eyes of 33 healthy controls who had never smoked were studied. The chronic smokers were at the start of the study and reevaluated at the end of the study after not smoking for 3 months.
Examination protocol and study measurements
One month before smoking cessation, each volunteer was given a comprehensive ophthalmic evaluation that included best-corrected visual acuity, slit lamp biomicroscopy, dilated stereoscopic fundus examination, intraocular pressure measurement by Goldmann applanation tonometry, and OCT imaging measurements. The same evaluation was repeated after 3 months of smoking cessation, and the results were compared with those obtained in the control group. Choroidal images were obtained with a Heidelberg Spectralis SD-OCT (Heidelberg Engineering, Heidelberg, Germany) equipped with an enhanced depth imaging module. Choroidal thickness was the vertical distance from the hyperreflective line of Bruch’s membrane to the hyperreflective line of the inner surface of the sclera. The choroidal thickness was measured at the subfovea, temporally at 500, 1,000, and 1,500 µm and nasally at 500, 1000, and 1,500 µm from the center of the fovea. The central foveal thickness was also measured. All OCT scans were performed in the morning to avoid diurnal fluctuations.
Exclusion criteria
Patients with a history of ocular disease that prevented examination of the cornea and retina, a history of ocular surgery, ocular or systemic diseases, a spherical refractive error >3 D, or a cylindrical refractive error >3 D were excluded.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences version 21.0 (SPSS Inc., Chicago, IL, USA). The normality of continuous variable distributions normality was checked with the Shapiro-Wilk test. If the differences in the foveal and choroidal measurements before and after smoking cessation were normally distributed, they were compared with the paired t-test. If not, the Wilcoxon signed-rank test was used. Differences in the mean values of independent groups were compared with the independent Student’s t-test or Mann-Whitney U test was used. Analysis of covariance (ANCOVA) was used to evaluate the significance of differences of initial and final participant evaluation measurements. The initial choroidal thickness results were covariates in the ANCOVA. Logarithmic transformation of nonparametric data was performed as needed to perform parametric analysis. Results were reported as means ± standard deviation and with 95% confidence intervals p≤0.05 were considered statistically significant.
RESULTS
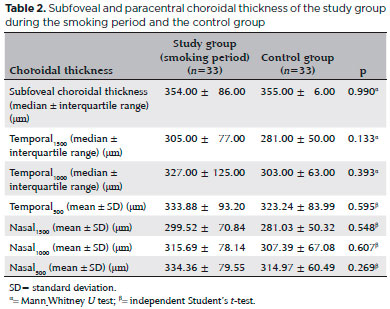
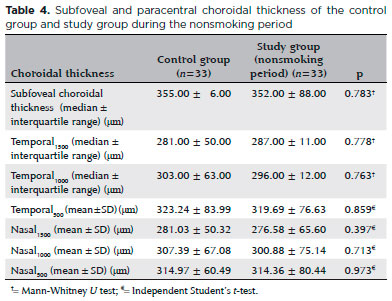
Thirty-three eyes of 33 chronic smokers (study group) and 33 eyes of 33 healthy controls who had never smoked were evaluated. The baseline ocular characteristics of the participant are summarized in table 1. The mean age of the smokers was 41.88 ± 6.52 years (range, 26-52), 23 (70%) were men, and 10 (30%) were women. The average smoking duration was 8.6 ± 2.5 years (range, 5-16). The subfoveal and paracentral choroidal thickness measurements in the study group smoking period and the control group are shown in table 2. The mean central macular thickness was 267.21 ± 18.42 µm in the smoking and 268.42 ± 18.28 µm in the nonsmoking period (p=0.022). The mean subfoveal and paracentral choroidal thickness values during the study group smoking and nonsmoking periods are shown in table 3. The paracentral choroidal thickness measurements were significantly lower than the baseline values after 3 months of smoking. The subfoveal choroidal thicknesses in the smoking and nonsmoking periods were not significantly different (p=0.194). The subfoveal and paracentral choroidal thickness measurements of the control and the study nonsmoking period are shown in table 4.
DISCUSSION
Central foveal thickness increased and parafoveal choroidal thickness decreased after smoking cessation. A change in subfoveal choroidal thickness has not previously been reported. Overall, the reported effects of cigarette smoking on choroidal thickness are inconsistent. A study by Kantarci et al.(14) reported that choroidal thickness in long-term smokers and in healthy participants was not significantly different, but other studies found that cigarette smoking caused short-term(15,16), but not long-term(16) changes. Others have reported decreases in choroidal thickness in chronic smokers(17,18). A study by Tamaki et al.(19) found that a decrease in tissue blood velocity at the optic nerve head and possibly in the choroid, suggesting a significant increase in vascular resistance in those tissues.
An animal study found that increased choroidal vascular resistance was associated with chronic exposure to tobacco smoke(20). Kaiser et al. reported increased flow rates in the ophthalmic artery, central retinal artery, and posterior ciliary arteries of chronic smokers(21), but others have reported decreased blood flow rates in chronic smokers(22,23).
A study of the effects of carbogen breathing revealed abnormal choroidal vascular responses in chronic smokers compared with nonsmokers(24).
Choroidal blood flow is responsive to pCO2(25), but the cause of vasodilation induced by CO2 is not clear. The reduction of nitrite to NO could lead to vasodilatation because of hypercapnia and reduced pO2(26), but NO-mediated vasodilation is impaired in smokers(27,28). The evidence suggests that the choroid is affected by smoking because of the presence of a rich capillary network. A recent study found that the central fovea was thinner in active than in passive smokers. In addition to affecting the structure of the macula, multifocal electroretinography showed that active smoking reduced macular functional responses(29). However, Kantarci et al.(14) reported that the central macular thickness did not differ in long-term smokers and healthy participants.
Nicotine replacement therapy, bupropion, and varenicline are medications indicated for smoking cessation. The US Preventive Services Task Force reported that intervention improved the likelihood of smoking cessation within 6 months or more(30), and a meta-analysis by Cahill et al.(31) reported that varenicline and bupropion were superior to a placebo in smoking cessation. In this single-center study, 33 of 150 chronic smokers with baseline measurements quit smoking. The study was limited by the small number of participants. They used medications to help stop cigarette smoking, but smoking cessation was self-reported. Despite its weaknesses, this study is valuable because it is the first to examine changes in the central foveal and choroidal thicknesses of patients who stopped smoking. In conclusion, subfoveal choroidal thickness did not change, central foveal thickness significantly increased, and parafoveal choroidal thickness was significantly decreased in patients who quit smoking.
REFERENCES
1. Müezzinler A, Mons U, Gellert C, Schottker B, Jansen E, Kee F, et al. Smoking and all-cause mortality in older adults: results from the CHANCES consortium. Am J Prev Med. 2015;49(5):e53-e63.
2. Hill C. [Tobacco epidemiology]. Rev Prat. 2012;62(3):327-9. French.
3. Solberg Y, Rosner M, Belkin M. The association between cigarette smoking and ocular diseases. Surv Ophthalmol. 1998;42(6):535-47.
4. Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR; Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47-55. Comment in: Expert Opin Pharmacother. 2006;7(18):2599-603; JAMA. 2006;296(21):2555; author reply 2555-6; Curr Psychiatry Rep. 2007;9(5):345-6; JAMA. 2006;296(1):94-5.
5. Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR; Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006; 296(1):56-63. Erratum in: JAMA. 2006;296(11):1355. Comment in: Curr Psychiatry Rep. 2007;9(5):346-7; JAMA. 2006;296(1):94-5; JAMA. 2006;296(21):2555;author reply 2555-6.
6. Mihalak K, Carroll FI, Luetje CW. Varenicline is a parietal agonist at {alpha]4{beta}2 and a full agonist at {alpha}7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70(3):801-5.
7. Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106-13.
8. Hurt RD, Sachs DP, Glover ED, Offord KP, Jonhston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195-202. Comment in: N Engl J Med. 1998;338(9):619; N Engl J Med. 1997; 337(17):1230-1; N Engl J Med. 1998;338(9):619-20.
9. Warner C, Shoaib M. How does bupropion work as a smoking cessation aid? Addict Biol. 2005;10(3):219-31.
10. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325-9.
11. Goldenberg D, Moisseiev E, Goldstein M, Loewenstein A, Barak A. Enhanced depth imaging optical coherence tomography: choroidal thickness and correlations with age, refractive error, and axial length. Ophthalmic Surg Lasers Imaging. 2012;43(4):296-301.
12. Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469-73. Comment in: Retina. 2010;30(8):1320-1.
13. Nishida Y, Fujiwara T, Imamura Y, Lima LH, Kurosaka D, Spaide RF. Choroidal thickness and visual acuity in highly myopic eyes. Retina. 2012;32(7):1229-36.
14. Kantarci FA, Tatar MG, Colak HN, Uslu H, Yildirim A, Goker H, et al. A pilot study of choroidal thickness in long-term smokers. Retina. 2016;36(5):986-91. Erratum in: Retina. 2017;37(11):e147.
15. Ulaş F, Çelik F, Doğan Ü, Çelebi S. Effect of smoking on choroidal thickness in healthy smokers. Curr Eye Res. 2014;39(5):504-11.
16. Sızmaz S, Küçükerdönmez C, Pınarcı EY, Karalezli A, Canan H, Yilmaz G. The effect of smoking on choroidal thickness measured by optical coherence tomography. Br J Ophthalmol. 2013;97(5):601-4.
17. Sigler EJ, Randolph JC, Calzada JI, Charles S. Smoking and choroidal thickness in patients over 65 with early-atrophic age-related macular degeneration and normals. Eye (Lond). 2014;28(7):838-46.
18. Moschos MM, Nitoda E, Laios K, Ladas DS, Chatziralli IP. The impact of chronic tobacco smoking on retinal and choroidal thickness in Greek population. Oxid Med Cell Longev. 2016;2016:2905789.
19. Tamaki Y, Araie M, Nagahara M, Tomita K, Matsubara M. The acute effects of cigarette smoking on human optic nerve head and posterior fundus circulation in light smokers. Eye (Lond). 2000;14(Pt 1): 67-72.
20. Hara K. [Effects of cigarette smoking on ocular circulation chronic effect on choroidal circulation]. Nippon Ganka Gakkai Zasshi. 1991; 95(10):939-43. Japanese.
21. Kaiser HJ, Schoetzau A, Flammer J. Blood flow velocity in the extraocular vessels in chronic smokers. Br J Ophthalmol. 1997; 81(2):133-5.
22. Steigerwalt RD Jr, Laurora G, Incandela L, Cesarone MR, Belcaro GV, De Sanctis MT. Ocular and orbital blood flow in cigarette smokers. Retina. 2000;20(4):394-7.
23. Williamson TH, Lowe GD, Baxter GM. Influence of age, systemic blood pressure, smoking, and blood viscosity on orbital blood velocities. Br J Ophthalmol. 1995;79(1):17-22.
24. Wimpissinger B, Resch H, Berisha F, Weigert G, Schmetterer L, Polak K. Response of choroidal blood flow to carbogen breathing in smokers and non-smokers. Br J Ophthalmol. 2004;88(6):776-81.
25. Geiser MH, Riva CE, Dorner GT, Diermann U, Luksch A, Schmetterer L. Response of choroidal blood flow in the foveal region to hyperoxia and hyperoxia-hypercapnia. Curr Eye Res. 2000; 21(2):669-76.
26. Iadecola C, Zhang F. Nitric oxide-dependent and-independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266(2 Pt 2):R546-52.
27. McVeigh GE, Lemay L, Morgan D, Cohn JN. Effects of long-term cigarette smoking on endothelium-dependent responses in humans. Am J Cardiol. 1996;78(6):668-72.
28. Butler R, Morris AD, Struthers AD. Cigarette smoking in men and vascular responsiveness. Br J Clin Pharmacol. 2001;52(2):145-9.
29. El-Shazly AA, Farweez YA, Elzankalony YA, Elewa LS, Farweez BA. Effect of smoking on macular function and structure in active smokers versus passive smokers. Retina. 2017. doi: 10.1097/IAE.0000000000001632.
30. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-20. Comment in: Lancet. 2016;387(10037):2481-2.
31. Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;31(5):CD009329.
Submitted for publication:
October 30, 2017.
Accepted for publication:
February 9, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose
Approved by the following research ethics committee: Kayseri Education and Research Hospital (# 50/2016)