

Kaline Sandrelli Amorim Ferreira1; Bruna Marília Alves dos Santos1; Nelise de Paiva Lucena1; Milena Sales Ferraz2; Rafaela de Siqueira Ferraz Carvalho2; Anivaldo Pereira Duarte Júnior2; Nereide Stela Santos Magalhães2; Rodrigo Pessoa Cavalcanti Lira1
DOI: 10.5935/0004-2749.20180090
ABSTRACT
Purpose: To determine the release profile of moxifloxacin encapsulated in liposomes in the aqueous humor as a controlled release system for intracameral application.
Methods: Liposomes containing moxifloxacin were obtained using the lipid film hydration method and were characterized by particle size and encapsulation efficiency. Female rabbits were used for the in vivo profile release study. Liposomes containing moxifloxacin was injected into the anterior chamber of the right eye of each animal. The rabbits were divided into five groups, and a sample of aqueous humor was collected 2, 4, 8, 24, and 48 h after administration of liposomes containing moxifloxacin administration. Moxifloxacin concentrations in the aqueous humor were analyzed using high-performance liquid chromatography.
Results: The average size of the liposomes containing moxifloxacin was 60.5 ± 0.72 nm with a particle size distribution of 0.307. The encapsulation efficiency of moxifloxacin in liposomes was 92.24 ± 0.24%. The results of an in vivo release study of liposomes containing moxifloxacin, showed that the maximum moxifloxacin concentration was achieved within the first 2 h after administration (5.27 ± 1.09 mg/mL) and was followed by a decrease in intracameral concentration (0.35 ± 0.05 mg/mL) until the 24 h mark.
Conclusions: The in vivo experiments resulted in liposomes containing moxifloxacin that were homogenous in size and exhibited high drug encapsulation efficiency. The results indicate that liposomes containing moxifloxacin offers a satisfactory aqueous humor release profile after intracameral application.
Keywords: Nanotechnology; Nanoparticles; Liposomes; Drug delivery system; Moxifloxacin; Endophthalmitis; Fluoroquinolones; Animals; Rabbit
RESUMO
Objetivo: Determinar o perfil de liberação, no humor aquoso, de moxifloxacino encapsulado em lipossomas como um sistema de liberação controlada para aplicação intracameral.
Métodos: Lipossomas contendo moxifloxacino foram obtidos através do método de hidratação do filme lipídico e caracterizados por tamanho da partícula e eficiência de encapsulação. Utilizaram-se coelhos fêmeas foram para o estudo do perfil de liberação in vivo. Lipossomas contendo moxifloxacino foram injetados na câmara anterior do olho direito de cada animal. Os coelhos foram divididos em cinco grupos, e uma amostra de humor aquoso foi coletada 2, 4, 8, 24 e 48 h após a administração de lipossomas contendo moxifloxacino. As concentrações de moxifloxacino no humor aquoso foram analisadas usando cromatografia líquida de alta eficiência.
Resultados: O tamanho médio dos lipossomas contendo moxifloxacino foi de 60,5 ± 0,72 nm com uma distribuição de tamanho de partícula de 0,307. A eficiência de encapsulação de moxifloxacino nos lipossomas foi de 92,24 ± 0,24%. Os resultados de um estudo de liberação in vivo de lipossomas contendo moxifloxacino, mostraram que a concentração máxima de moxifloxacino foi atingida dentro das primeiras 2 h após sua administração (5,27 ± 1,09 mg/mL) e foi seguida de um decréscimo na concentração intracameral (0,35 ± 0,05 mg/mL) até a marca de 24 h.
Conclusão: Os experimentos in vivo resultaram em lipossomas contendo moxifloxacino que eram homogêneos em tamanho e exibiam alta eficiência de encapsulação do fármaco. Os resultados indicam que lipossomas contendo moxifloxacino oferecem um perfil de liberação de humor aquoso satisfatório após a aplicação intracameral.
Descritores: Nanotecnologia; Naniopartículas; Lipossomos; Sistemas de liberação de medicamentos; Endoftalmite; Fluoroquinolonas; Animais; Coelhos
INTRODUCTION
Endophthalmitis is a serious complication of cataract surgery, with an incidence ranging from 0.014% to 1.238%(1), which can result in vision loss and debilitation. Coagulase-negative staphylococci, streptococci, and other staphylococci are the most common causes of endophthalmitis, whereas Gram-negative and anaerobic bacteria are less frequent etiologic agents(1).
Because endophthalmitis is a serious condition, prophylactic measures have been proposed that include the use of topical antibiotics in the preoperative and postoperative periods, antiseptics such as povidone-iodine at the time of surgery, and careful preparation of the surgical field. However, the information in the literature regarding the choice of prophylactic interventions in cataract surgery is rather limited(1). The guidelines of the European Society of Cataract and Refractive Surgeons for the prevention and treatment of endophthalmitis after cataract surgery report that intracameral prophylactic use of antibiotics may reduce the incidence of postoperative endophthalmitis by up to 25%(1). The most commonly used antibiotics for treatment of postoperative endophthalmitis are vancomycin and cefuroxime. However, these drugs need to be prepared for intracameral injection, and errors in preparation have been associated with some adverse effects, such as toxic anterior segment syndrome(2,3).
Intracameral moxifloxacin (MFLX) has been used off label for the prevention of post-cataract endophthalmitis(3). However, intracameral MFLX is limited by aqueous humor (AH) turnover.
In order to improve the ophthalmologic bioavailability of MFLX, the use of drug delivery systems, such as liposomes, has been proposed. Liposomes are small and spherical vesicles composed of membrane-like lipid bilayers surrounding aqueous compartments. Liposomes are used to extend therapeutic effects by prolonging drug bioavailability at the site of action. Furthermore, liposomes can reduce toxicity by decreasing the peak drug concentration(4). Several studies in the field of liposomal ocular delivery have been proposed(5-9), but there has been no study on liposome delivery of MFLX.
The purpose of this study was to assess the release profile of MFLX-loaded liposomes in the AH and the use of liposomes as a prolonged release system for intracameral application.
METHODS
Setting
The study was conducted at the Laboratory of Immunopathology Keizo Asami and Nucleus of Experimental Surgery of the Federal University of Pernambuco.
Design
This was an experimental animal study.
High-performance liquid chromatography assay of MFLX
A simple and highly sensitive isocratic high-performance liquid chromatography (HPLC) assay was used to determine MFLX concentrations. The chromatographic analysis was performed using an Alliance 2695 HPLC system (Waters, Milford, MA, USA) coupled to a PDA 2998 photodiode array (Waters Corporation) operating at 296 nm. A C18 reversed-phase column (250 mm × 4.6 mm, 5 mm, XBridgeTM; Waters Corporation) protected by a guard column of the same composition (20 mm × 4.6 mm) was used in the mobile phase composed of acidified water (0.1% trifluoroacetic acid), acetonitrile, and methanol (55:30:15 v/v/v) at a flow rate of 1 mL/min at 50 °C and an injection volume of 20 µL. The peak MFLX retention time was recorded at 4.1 min. MFLX standards were obtained from Sigma-Aldrich Corporation (St. Louis, MO, USA), dissolved in acidified water, and injected in every analysis. An MFLX analytical curve (0.5-20 mg/mL) was prepared from dilutions of a stock solution. The correlation coefficient obtained from analysis of six standard samples was 0.9996.
Liposome preparation
Conventional liposomes containing MFLX (MFLX-Lipo) were obtained using the lipid film hydration method proposed by Lira et al.(10) with minor modifications. First, MFLX was solubilized in 2 mL of methanol. Next, the lipids (60 mM)-soy phosphatidylcholine, cholesterol, and stearylamine (70:20:10)-were solubilized separately in a chloroform: methanol (3:1) solution. All components were mixed together, and the resulting solution was placed into a rotary evaporator under reduced pressure until a uniform film was formed. The lipid film was hydrated with 5 mL of phosphate-buffered saline (pH 7.4) to obtain a formulation of 1 mg/mL. The solution was subjected to an ultrasound probe (Vibra Cell Ultrasonic Processor; Sonics & Materials, Inc., Newtown, CT, USA) at 200 W and 40 Hz for 300 s to obtain small unilamellar liposomes.
Characterization of liposomes
MFLX-Lipo particle size and distribution
The mean particle size and polydispersity index of each liposomal suspension were determined using photon correlation spectroscopy with a DelsaNanoTM laser-S particle analyzer (Beckman Coulter, Brea, CA, USA).
Encapsulation efficiency of MFLX-Lipo
The encapsulation efficiency was determined by ultrafiltration and ultracentrifugation techniques and Microcon® filter units (EMD Millipore, Burlington, MA, USA), in which MFLX-Lipo samples (500 µL) were centrifuged (Ultracentrifuge KT-20000; KUBOTA Corporation, Osaka, Japan) at 14,000 rpm for 30 min at 4°C. The ultrafiltrate concentration of MFLX (free drug) was determined by HPLC. Finally, the encapsulation efficiency (%) was calculated as the difference between the total concentration of encapsulated drug in the suspension and that of the non-encapsulated drug (free).
In vivo study of the MFLX-Lipo release profile
Animal model
Fifteen female New Zealand rabbits, weighing 2.5-4.8 kg and free of any gross ocular defects, were selected for the in vivo study. The protocol of the animal study was approved by the Ethics Committee on Animal Experimentation of the Federal University of Pernambuco, Brazil (CAAE Registry No. 23076.011824/2015-11) and conducted in accordance with the ethical principles for animal experimentation developed by the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Study design
First, 0.05 mL of MFLX-Lipo (1 mg/mL) was injected into the anterior chamber of the right eye of each rabbit. The rabbits were divided into five groups of three rabbits each. An AH sample (0.1 mL) was collected from the right eye of each rabbit using a 30G needle after 2 (group 1), 4 (group 2), 8 (group 3), 24 (group 4), and 48 h (group 5) of follow-up. The AH samples were frozen in liquid nitrogen for subsequent analysis. One thawed, the AH samples (50 µL) were diluted with acetonitrile, homogenized, briefly vortex mixed for 1 min, filtered (Millex® filters, 0.22 mm; EMD Millipore), and injected into the HPLC system. The MFLX concentrations in the AH samples (free drug) were determined through the calculation of the area of the chromatographic peak relative to the standard peak and by comparison of this ratio with standards with known amounts of the antibiotic.
RESULTS
The average size of MFLX-Lipo was 60.5 ± 0.72 nm with a fair and monodisperse size distribution (polydispersity index = 0.307). According to the HPLC results, the encapsulation efficiency of MFLX into liposomes was greater than 90% (92.24 ± 0.24%).
In the current study, no ocular complications, systemic complications, or adverse events were observed. The concentrations of MFLX (µg/mL) released from liposomes over time and obtained from AH samples are shown in figure 1. The maximum MFLX concentration was achieved in the first 2 h after administration (5.27 ± 1.09 mg/mL) and was followed by a decrease in intracameral concentration (0.35 ± 0.05 mg/mL) until the 24 h mark. At this point, the intracameral concentration remained constant until the study was concluded at the 48 h mark.
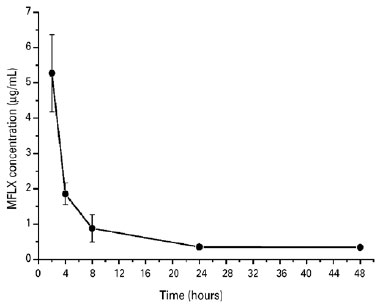
DISCUSSION
The liposomal formulation was found to be stable and homogeneous. The size distribution and polydispersity index of liposomal vesicles are important parameters that indicate the uniformity and homogeneity of the formulation. In addition, these parameters can influence drug loading, release, and in vivo distribution. The greater the curvature of the lipid bilayer (the lower the liposomal vesicle), the more unstable the liposomal vesicle and, theoretically, the lower the encapsulation efficiency(11). In the literature, liposome formulations used for intraocular administration had an average diameter of 100 to 400 nm(12). In this study, the liposome particle size was very small at 60.5 ± 0.72 nm, suggesting that the sonication time should be decreased in future studies to obtain particles with an average diameter greater than 100 nm.
The challenge in the development of liposomes as ocular drug delivery systems is to provide a system with improved drug bioavailability and activity duration, but with a minimum risk of ocular complications. The liposomal encapsulation efficiency is one of the main factors influencing the therapeutic effect of vesicular dosage forms(13). In this study, MFLX-Lipo exhibited high encapsulation efficiency.
The literature reports that drug release profiles from liposomes tend to show an initial rapid rate of drug loss, which then slows. The initial rapid release rate is commonly ascribed to drug detachment from the liposomal surface, whereas the later slow release rate results from sustained drug release from the inner lamellae(14). The in vivo release study of MFLX-Lipo revealed a rapid release rate in the first 8 h with a slower release profile over the next 40 h. Although the in vivo release profile of MFLX-Lipo was consistent with the literature, a slower drug release rate was expected. In that regard, in future studies, a modification in the cholesterol ratio of the formulation is suggested to alter membrane fluidity and stiffness, which will possibly decrease the drug release rate and increase ocular bioavailability.
In a recent investigation of the ocular pharmacokinetics, safety, and efficacy of MFLX in a rabbit model(15), 0.1 mL of 0.5% MFLX (500 µg) was injected into the right eyes of New Zealand rabbits. The MFLX concentration in the AH samples until the 24 h mark was 0.068 ± 0.009 µg/mL. In the present study, 0.05 mL of liposomes loaded with MFLX 0.1% (50 µg) was injected to the right eyes of 15 New Zealand rabbits. Even at an initial concentration 10-fold lower than that of a previous study, the MFLX concentration in the AH samples until the 24 h mark was 0.34 ± 0.06 µg/mL (5-fold higher concentration).
Although these results are promising, there were many limitations to this experimental study. New formulations need to be developed to guarantee a higher concentration, longer half-life, and more constant intracameral drug release.
In summary, the in vivo experiments showed that liposomes containing MFLX were homogenous in size and dispersion and exhibited high drug encapsulation efficiency. The results indicate that MFLX-Lipo offers a satisfactory AH release profile after intracameral application.
REFERENCES
1. Barry P, Cordovés L, Gardner S. ESCRS Guidelines on prevention and treatment of endophthalmitis following cataract surgery: data, dilemmas and conclusions [Internet]. Dublin: European Society of Cataract and Refractive Surgeons; 2014. p.1-16. [cited 2017 nov 21]. Available from: http://www.escrs.org/downloads/Endophthalmitis-Guidelines.pdf
2. Filip M, Dragne C, Filip A, Magureanu M, Zemba M, Asandi R. [Toxic anterior segment syndrome]. Oftalmologia. 2006;50(4):27-9.
3. Braga-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014; 40(12):2134-42.
4. Dale M, Michael M. Liposome ocular delivery systems. Adv Drug Delivery Rev. 1995;1(16):75-93.
5. Singh K, Mezei M. Liposomal ophthalmic drug delivery system. I. Triamcinolone acetonide. Int J Pharm. 1983;16:339-44.
6. Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery. III. Pharmacodynamic and biodisposition studies of atropine. Int J Pharm. 1989;55:105-13.
7. Fishman PH, Peyman GA, Lesar T. Intravitreal liposome-encapsulated gentamicina in a rabbit model. Prolonged therapeutic levels. Invest Ophthalmol Vis Sci. 1986;27(7):1103-6.
8. Peyman GA, Khoobehi B, Tawakol M, Schulman JA, Mortada HA, Alkan H, et al. Intravitreal injection of liposome-encapsulated ganciclovir in a rabbit model. Retina. 1987;7(4):227-9.
9. Alghadyan AA, Peyman GA, Khoobehi B, Liu KR. Liposome-bound cyclosporine: Retinal toxicity after intravitreal injection. Int Ophthalmol. 1988;12(2):105-7.
10. Lira MC, Ferraz MS, Da Silva DG, Cortes, ME, et al. J Incl Phenom Macrocycl Chem. 2009;64:215-24.
11. Bochot A, Fattal E. Liposomes for intravitreal drug delivery: A state of the art. J Control Release. 2012;161(2):628-34.
12. Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: a shift to the posterior segment. Drug discovery Today. 2008;13(3):135-43.
13. Kaszás N, Budai M, Budai L, Gróf P, Zimmer A, Klebovich I. [Methods to increase the encapsulation efficiency for liposomal drugs]. Acta Pharm Hung. 2008;78(2):69-74. Hungarian.
14. Henriksen I, Sande SA, Smistad G. In vitro evaluation of drug release kinetics from liposomes by fractional dialysis. Int J Pharm. 1995;119:231-8.
15. Asena L, Akova YA, Goktas MT, Bozkurt A, Yasar U, Karabay G, et al. Ocular pharmacokinetcs, safety and efficacy of intracameral moxifloxacin 0,5% solution in a rabbit model. Curr Eye Res. 2013; 38(4):472-9.
Submitted for publication:
October 10, 2017.
Accepted for publication:
March 13, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose
Approved by the following research ethics committee: Use of Animals of Universidade Federal de Pernambuco (case # 23076.011824/2015-11, letter # 78/15)