

Burcin Kepez Yildiz1,2; Zeynep Aktas1; Nilay Yuksel3; Huseyin Baran Ozdemir1,4; Mehmet Cingirt5; Ozlem Gulbahar5; Fulya Yaylacioglu Tuncay1,6; Murat Hasanreisoglu1
DOI: 10.5935/0004-2749.20180092
ABSTRACT
Purpose: Pseudoexfoliation syndrome has been linked to impaired function of the heart and blood vessels. We conducted a study to investigate the role of the renin-angiotensin system in the etiopathogenesis of pseudoexfoliation syndrome.
Methods: The subjects were 14 patients with pseudoexfoliation syndrome and 14 healthy controls who underwent cataract extraction. Preoperative 5-ml samples of peripheral venous blood and perioperative aqueous humor were collected from the patients in both groups. Plasma and aqueous humor renin levels were analyzed by an immunoradiometric method, and angiotensin II levels were analyzed by radioimmunassay. SPSS version 16.0 was used for statistical analyses. A p-value <0.05 was considered to indicate a statistically significant difference.
Results: The mean ages of the patients in pseudoexfoliation and control groups were 71.7 ± 7.1 and 67.4 ± 9.3 years, respectively (p=0.140). The median aqueous humor renin level was 7.73 pg/ml (4.15-21) in the control group and 11.95 pg/ml (3.75-18.54) in pseudoexfoliation group (p=0.022). There were no differences between the two groups in the plasma renin, plasma angiotensin II, or aqueous humor angiotensin II levels. The correlations between plasma and aqueous humor renin levels and between plasma and aqueous humor angiotensin II levels were examined separately for each group; no significant correlations were observed in pseudoexfoliation group (r=-0.440, p=0.115; r=-0.414, p=0.142) or the control group (r=-0.232, p=0.425; r=0.482, p=0.081).
Conclusion: Aqueous humor renin levels are higher in pseudoexfoliation syndrome. The results indicate a probable role of renin-angiotensin system in pseudoexfoliation syndrome. Further studies with larger numbers of cases are needed to clarify the precise association of renin-angiotensin system with the etiopathogenesis of pseudoexfoliation syndrome.
Keywords: Exfoliation syndrome/etiology; Renin-angiotensin system; Receptor, angiotensin, type 2; Peripheral vascular diseases
RESUMO
Objetivo: A síndrome de pseudo-exfoliação tem sido associada ao comprometimento da função do coração e dos vasos sanguíneos. Foi realizado um estudo para investigar o papel do sistema renina-angiotensina na etiopatogenia da síndrome de pseudo-exfoliação.
Métodos: Os sujeitos foram 14 pacientes com síndrome de pseudo-exfoliação e 14 controles saudáveis submetidos à extração de catarata. Amostras pré-operatórias de 5 ml de sangue venoso periférico e humor aquoso perioperatório foram coletadas dos pacientes em ambos os grupos. Os níveis de renina no plasma e humor aquoso foram analisados pelo método imunorradiométrico e os níveis de angiotensina II foram analisados por radioimunoensaio. O SPSS versão 16.0 foi utilizado para análises estatísticas. Considerou-se o valor de p<0,05 para indicar uma diferença estatisticamente significativa.
Resultados: A média de idade dos pacientes nos grupos pseudo-exfoliação e controle foi de 71,7 ± 7,1 e 67,4 ± 9,3 anos, respectivamente (p=0,140). O nível médio de renina no humor aquoso foi de 7,73 pg / ml (4,15-21) no grupo controle e 11,95 pg/ml (3,75-18,54) no grupo pseudo-exfoliação (p=0,022). Não houve diferenças entre os dois grupos de renina plasmática, angiotensina II plasmática ou nos níveis de angiotensina II em humor aquoso. As correlações entre os níveis de renina no plasma e no humor aquoso e entre os níveis de angiotensina II no plasma e humor foram examinadas separadamente para cada grupo; n]ao foram observadas correlações significativas no grupo pseudo-exfoliação (r=-0,440, p=0,115; r=-0,414, p=0,142) ou no grupo controle (r=-0,232, p=0,425; r=0,482, p=0,081).
Conclusão: Os níveis de renina no humor aquoso são mais elevados na síndrome de pseudo-exfoliação. Os resultados indicam um provável papel do sistema renina-angiotensina na síndrome de pseudo-exfoliação. Novos estudos com maior número de casos são necessários para esclarecer a associação precisa do sistema renina-angiotensina com a etiopatogenia da síndrome de pseudo-exfoliação.
Descritores: Síndrome de exfoliação/etiologia; Sistema reninaangiotensina; Receptor tipo 2 de angiotensina; Doenças vasculares periféricas
INTRODUCTION
Pseudoexfoliation (PEX) syndrome is a common age-related fibrillopathy of unknown cause. It is characterized by the deposition of distinctive fibrillar material in the anterior segment(1). PEX syndrome is not only an ocular disease but also a generalized disorder that involves the abnormal production of extracellular matrix material(2).
Recent studies suggest that PEX syndrome is frequently linked to impaired function of the heart and blood vessels. Systemic and ocular blood flow changes, altered parasympathetic vascular control, increased vascular resistance, and decreased blood flow velocity, high levels of plasma homocysteine, and arterial hypertension have all been demonstrated in patients with PEX syndrome(3). Furthermore, endothelin-1, a potent vasoconstrictor, seems to be increased in the aqueous humor of PEX eyes(4). Although deposition of pseudoexfoliative fibers in the cardiovascular system and the relation of PEX syndrome to coronary artery disease and cardiovascular mortality have been documented, data about the significance of the relationship are limited and conflicting(5,6). Vascular injury, characterized by endothelial dysfunction, structural remodeling, inflammation, and fibrosis, plays an important role in cardiovascular diseases(7). Among the factors involved in arterial remodeling, angiotensin II appears to be one of the most important factors(8). Here the probable role of angiotensin II in the PEX syndrome is a matter of wonder.
The renin-angiotensin system (RAS) is an enzymatic cascade that generates a range of angiotensin peptides with various biological actions. The circulating RAS, through the production of its biological effector angiotensin II, has an important role in the control of electrolyte homeostasis, renal hemodynamics, and blood pressure(9).
Independently of systemic RAS, tissue intrinsic RASs have been identified in various tissues, including the retina, and are important for maintaining local homeostasis(10). Although intraocular RAS has been identified in ocular tissues, its precise function has not been established.
Many RAS components have been shown in cultured, human, nonpigmented ciliary body epithelium in those cells that are particularly responsible for aqueous humor formation(11). RAS expression is present in the trabecular meshwork, which is involved in aqueous humor outflow(12). Thus the RAS plays a role in regulating aqueous humor dynamics and thus intraocular pressure (IOP). Furthermore, inflammatory diseases such as uveitis, diabetic retinopathy, and age-related macular degeneration have been shown to be associated with this local RAS(10).
Inappropriate activation of the RAS is being increasingly recognized as a major factor in determining endothelial dysfunction, arterial stiffness, and progression to cardiovascular diseases(13,14). There are still many questions that have to be answered about PEX syndrome, but cardiovascular morbidity and mortality may give clues to its pathogenesis. Although there has been growing interest in the RAS in ocular tissues and ocular diseases, to our knowledge, the involvement of local or systemic RAS in PEX syndrome has not been studied. The aim of this study is to investigate the possible role of the RAS in PEX syndrome without glaucoma.
METHODS
This prospective study was conducted between January 2013 and June 2013 in the Ophthalmology and Biochemistry Departments of the Gazi University School of Medicine. It was performed in accordance with the Declaration of Helsinki of the World Medical Association and was approved by the local ethics committee. After informed consent was obtained from the patients, 14 patients with PEX syndrome with cataracts and 14 healthy control patients with only cataracts who visited the Ophthalmology Department were included in the study. Both undilated and dilated slit-lamp biomicroscopy were performed during the clinical examination to detect the white dandruff-like material on the pupil, lens, and the angle, as well as other signs such as parapupillary transillumination defects(15).
All patients had best corrected visual acuity <20/50 measured by Snellen chart, and phacoemulsification surgery with foldable intraocular lens implantation was planned. All participants had grade 3 cataract density according to the Lens Opacities Classification System III(16).
The inclusion criteria for the participants were IOP <21 mmHg in revised and randomized measurements; normal optic disc morphology with no obvious cupping or peripapillary atrophy; normal retinal nerve fiber layer thickness parameters measured by Heidelberg retinal angiography; no history of systemic hypertension, diabetes mellitus, or coronary heart disease; and no ocular comorbidities such as uveitis, diabetic retinopathy, or age-related macular degeneration. None of the patients or controls had a history of any systemic drug use.
To clarify the relation between aqueous renin-angiotensin production and circulating blood renin-angiotensin levels, we compared the levels in aqueous humor and plasma. Venous blood samples were obtained from the antecubital vein just before the start of phacoemulsification with the patient in the supine position in the operating room. The samples were centrifuged within 15 min of collection at 2.750 g for 10 min, and the supernatant serum was then transferred into polypropylene tubes. The samples were kept at -80°C in a freezer until they were analyzed. During the cataract surgery, 0.1-0.2 ml of aqueous humor samples was taken by tuberculin injectors before viscoelastic agent was introduced to the anterior chamber. The samples were obtained carefully to avoid touching the intraocular tissues or blood contamination. The aqueous humor samples were also kept in the freezer at -80°C. All samples were carefully protected from light and were sent immediately to the laboratory. Standard surgical procedure was followed for cataract extraction by phacoemulsification.
Biochemical analyses were performed in the Gazi University Biochemistry Department. Plasma and aqueous humor renin levels were analyzed by the immunoradiometric method with Beckman Coulter testing kits (IM 3518). We took great care to centrifuge blood within 30 min of collection, preferably within 10 min, and the plasma sample was rapidly frozen. Frozen plasma was rapidly thawed before assay and only once. Angiotensin II levels were analyzed by radioimmunassay with Dia Source testing kits (KIPERB320).
Statistical analyses
SPSS version 16.0 for personal computer was used for statistical analyses. Continuous data are given as means ± SD; categorical variables are given as numbers of cases and percentages. The significance of the difference between means was tested by Student’s t-test or the nonparametric Mann-Whitney U test when appropriate; the chi-square test was used to test for differences related to gender. Related samples were compared by the Wilcoxon test. Degrees of association between continuous variables were evaluated by Spearman’s rank correlation analyses. A p-value <0.05 was considered to indicate a statistically significant difference. The sample size was estimated using the free-software G*Power 3.1.9.2 (Franz Faul, University of Kiel, Kiel, Germany)(17). With a power of 80%, a 0.05 statistical level of significance, and an effect size of 1.055, the sample size for each group was calculated to be 10.
RESULTS
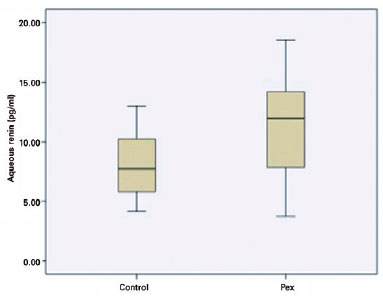
There were 10 men and 4 women in the PEX syndrome group and 8 men and 6 women in the control group (p=0.257). The mean ages of the patients in the PEX syndrome and control groups were 71.7 ± 7.1 years (56-82) and 67.4 ± 9.3 years (54-80), respectively (p=0.140). The mean preoperative IOP was 13.2 ± 2.9 mmHg in the PEX syndrome group and 15.4 ± 3.1 mmHg in the control group (p=0.670). The median aqueous humor renin level was 7.73 pg/ml (4.15-13) in the control group and 11.95 pg/ml (3.75-18.54) in the PEX syndrome group (Figure 1). The difference in aqueous humor renin levels was statistically significant (p=0.022). There was no difference in plasma renin levels between the two groups; the median plasma renin level was 7.5 pg/ml (2-33) in the control group and 8.5 pg/ml (1-22) in the PEX syndrome group (p=0.534). The median aqueous humor angiotensin II levels were 30.25 pmol/L (20.6-34.9) in the control group and 30.25 pmol/L (17.6-36.5) in the PEX syndrome group (p=0.520). In contrast, the median plasma angiotensin II levels were 34.7 pmol/L (10.9-91.0) and 26.63 pmol/L (9.8-120) in the control and PEX syndrome groups, respectively (p=0.395; Table 1).
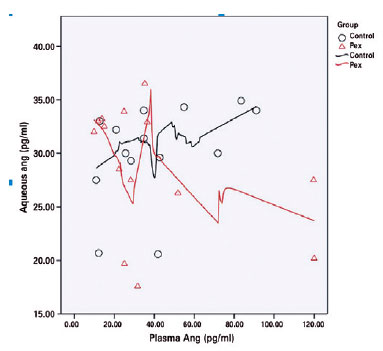
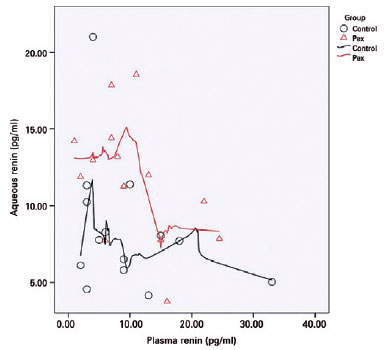
There was no correlation between the aqueous humor and the plasma renin levels in the control (r=-0.232, p=0.425) or the PEX syndrome (r=-0.440, p=0.115) groups. There was also no correlation between the aqueous humor and the plasma angiotensin II levels in the control (r=0.482, p=0.081) or the PEX syndrome (r=-0.414, p=0.142) groups (Figures 2 and 3).
DISCUSSION
PEX syndrome is a systemic disease characterized by the presence of dust-like PEX material in the ocular tissues, with a reported worldwide prevalence of 1.8%-13.5%(18). Although inflammation is also considered a factor in the etiopathogenesis of PEX syndrome, there is no evidence suggesting the RAS is involved. Taube et al.(19) reported lower levels of angiotensinogen in the aqueous humor of pseudoexfoliative eyes than in controls. Angiotensinogen is a substrate in the RAS. It may be speculated that angiotensinogen is decreased because of the excess of renin and is converted into angiotensin II. Tekeli et al.(20) investigated the relation between angiotensin-converting enzyme (ACE) gene polymorphism and PEX and reported that this gene does not play a role in the pathogenesis of PEX syndrome.
The RAS in vascular tissue is recognized as an important element involved in endothelial dysfunction(13,14). There is increasing evidence about cardiovascular mortality, impaired endothelial function, and PEX syndrome. The objective of our study was to assess the role of the RAS in PEX syndrome by comparing the aqueous humor and plasma levels of RAS components. Although its exact function is unknown, evidence now suggests the presence of a local RAS in the human eye(21). Specifically, RAS components have been identified in the human ciliary body and aqueous humor(22,23). Ho et al.(24) argued the opposite, that in most human studies, orally administered ACE inhibitors or angiotensin II receptor blockers have reduced IOP in normotensive and glaucomatous patients(25,26). The probable mechanism is considered to be increased outflow of aqueous humor in acute and chronic models of glaucoma in rabbits.
ACE inhibitors also reduce IOP by lowering the production of aqueous humor by reducing blood flow in the ciliary body(27). ACE inhibitors also prevent the breakdown of bradykinin and promote the synthesis of prostaglandins, which can lower IOP by increasing uveoscleral outflow(28). In addition to its probable ocular hypotensive effect, blockade of the ocular RAS may exert a neuroprotective effect in glaucoma, since angiotensin-induced vasoconstriction of ocular blood vessels is considered a pathogenic mechanism in optic nerve damage(29).
The finding that topical administration of ACE and renin inhibitors lowers IOP in patients with ocular hypertension and primary open glaucoma points to the potential role of this system in aqueous humor dynamics(30,31). Renin is expressed in the pigment epithelium and in retinal Müller cells(20). Angiotensinogen is the sole precursor of angiotensin peptides and is cleaved to generate angiotensin I by renin and aspartyl proteases. Angiotensin II, the main effector peptide of the RAS, can be liberated from angiotensin I by ACE or serine proteases(32). We studied the main components of the renin and angiotensin II pathway in both plasma and aqueous humor. We could not find any differences between PEX syndrome and control subjects in the levels of plasma renin, plasma angiotensin II, or aqueous humor angiotensin II. The only difference was in aqueous humor renin levels, which were higher in the PEX syndrome group (p=0.022). This might be interpreted as RAS activation in PEX syndrome. Increase in renin may be the first step of the cascade, because plasma levels of these mediators are similar in control and PEX syndrome subjects. In contrast, we cannot exclude the possibility that their roles may also be related to inflammatory responses.
The major drawback of our study is that our sample size was too small. Further studies will be important to test our hypothesis. Another issue of interest is how these components vary in pseudoexfoliative glaucoma. Another limitation is that the control group was younger than the PEX syndrome group, although the difference was not statistically significant. Since the prevalence of PEX syndrome increases with age, and PEX syndrome is a continuous progressive disease, we cannot exclude the possibility of controls to reveal PEX syndrome in the next decades. This condition may be conflicting for determining the actual correlation of biochemical biomarkers with PEX syndrome.
We looked for a correlation between plasma and aqueous humor levels of renin and angiotensin II within the PEX syndrome and control groups separately to determine whether the levels originated from local production or from the blood component, and we could not find any correlation. These results demonstrated the presence of renin and angiotensin II in the eye but did not exclude either their sequestration in the eye or local production. Osusky et al.(33) compared the plasma and aqueous humor levels of angiotensin II between untreated normotensive patients and patients with arterial hypertension who were taking either diuretics, which stimulate the RAS, or ACE inhibitors, which reduce the production of angiotensin II. Untreated normotensive patients who underwent cataract extraction had higher plasma levels of angiotensin II (2.28 ± 0.32 fmol/ml) than aqueous humor levels of the measurable ones (median <0.55 fmol/ml). In contrast, Danser et al.(31), studying porcine eyes, reported 5- to 100-fold higher levels of angiotensin II in ocular fluid than in plasma and interpreted this as confirming the existence of tissue RAS in the eye. Moreover, if the blood-retina barrier is intact, circulating angiotensin cannot reach the vitreous fluid, but if the barrier is disrupted, this becomes possible(29). Küchle et al.(34) reported that the blood-aqueous barrier was impaired in PEX syndrome. This might explain why plasma and aqueous humor levels of renin and angiotensin II were similar in pseudoexfoliative eyes in our study, but it should be kept in mind that these levels were similar in the control group as well. Therefore, our study is insufficient to prove the presence of a local RAS in the eye.
In conclusion, PEX syndrome is the most common identifiable cause of secondary open-angle glaucoma, but its etiopathogenesis is unclear(35). We found higher levels of renin in the aqueous humor of patients with PEX syndrome, and we wonder if this finding may be a sign of RAS activation in PEX syndrome. If the relationship between the RAS and PEX syndrome is confirmed by other evidence, this knowledge could provide different therapeutic options and prevent the formation of pseudoexfoliative glaucoma, which is one of the most difficult types of glaucoma to manage(36). Although our study demonstrated a minor role, further prospective studies with increased numbers of patients are needed to identify the probable relationship between the RAS and PEX syndrome.
REFERENCES
1. Emiroglu MY, Coskun E, Karapinar H, Capkın M, Kaya Z, Kaya H, et al. Is pseudoexfoliation syndrome associated with coronary artery disease? N Am J Med. 2010;2(10):487-90.
2. Praveen MR, Shah SK, Vasavada AR, Diwan RP, Shah SM, Zumkhawala BR, et al. Pseudoexfoliation as a risk factor for peripheral vascular disease: a case-control study. Eye (Lond). 2011;25(2):174-9.
3. Andrikopoulos GK, Alexopoulos DK, Gartaganis SP. Pseuodoexfoliation syndrome and cardiovascular diseases. World J Cardiol. 2014;6(8):847-54.
4. Koliakos GG, Konstas AG, Schlötzer- Schrehardt U, Hollo G, Mitova D, Kovatchev D, et al. Endothelin-1 concentration is increased in the aqueous humour of patients with exfoliation syndrome. Br J Ophthalmol. 2004;88(4):523-7.
5. Mitchell P, Wang JJ, Smith W. Association of pseudoexfoliation syndrome with increased vascular risk. Am J Ophthalmol. 1997; 124(5):685-7.
6. Shrum KR, Hattenhauer MG, Hodge D. Cardiovascular and cerebrovascular mortality associated with ocular pseudoexfoliation. Am J Ophthalmol. 2000;129(1):83-6.
7. Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16(6):431.
8. Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp Physiol. 2005;90(4):449-55.
9. Oparil S, Haber E. The renin-angiotensin system (second of two parts). N Engl J Med. 1974;291(9):446-57.
10. Kurihara T, Ozawa Y, Ishida S, Okano H, Tsubota K. Renin-angiotensin system hyperactivation can induce inflammation and retinal neural dysfunction. Int J Inflam. 2012:581695.
11. Cullinane AB, Leung PS, Ortego J, Coca-Prados M, Harvey BJ. Reninangiotensin system expression and secretory function in cultured human ciliary body non-pigmented epithelium. Br J Ophthalmol. 2002;86(6):676-83.
12. Vaajanen A, Vapaatalo H. Local ocular renin- angiotensin system- A target for glaucoma therapy? Basic Clin Pharmacol Toxicol. 2011; 109(4):217-24.
13. Milan A, Tosello F, Fabbri A, Vairo A, Leone D, Chiarlo M, Covella M, Veglio F. Arterial stiffness: from physiology to clinical implications. High Blood Press Cardiovasc Prev. 2011;18(1):1-12.
14. Mahmud A, Feely J. Arterial stiffness and the renin-angiotensinaldosterone system. J Renin Angiotensin Aldosterone Syst. 2004; 5(3):102-8.
15. Prince AM, Ritch R. Clinical signs of the pseudoexfoliation syndrome. Ophthalmology. 1986;93(6):803-7.
16. Chylack LT Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The lens opacities classification system III. The Longitudinal study of Cataract Study Group. Arch Ophthalmol. 1993; 111(6):831-6.
17. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149-60.
18. Naumann GOH, Schlötzer-Schrehardt U, Küchle M. Pseudoexfoliation syndrome for the comprehensive ophthalmologist; intraocular and systemic manifestations. Ophthalmology. 1989;105(6):951-68.
19. Taube AB, Hardenborg E, Wetterhall M, Artemenko K, Hanrieder J, Andersson M, et al. Proteins from aqueous humor of cataract patients with and without pseudoexfoliation syndrome. Eur J Mass Spectrom (Chichester). 2012;18(6):531-41.
20. Tekeli O, Turaçlı ME, Altınok B, Akar N, Elhan AH, No relation between angiotensin-converting enzyme gene polymorphism and pseudoexfoliation. Ophthalmic Res. 2008;40(1):32-4.
21. Wagner J, Jan Danser AH, Derkx FH, de Jong TV, Paul M, Mullins JJ, et al. Demonstration of renin mRNA, angiotensinogen mRNA, and angiotensin converting enzyme mRNA expression in the human eye: evidence for an intraocular renin-angiotensin system. Br J Ophthalmol. 1996;80(2):159-63. Comment in: Br J Ophthalmol. 1996;80(2):99-100.
22. Ferrari-Dileo G, Ryan JW, Rockwood EJ, Davis EB, Anderson DR. Angiotensin- converting enzyme in bovine, feline and human ocular tissues. Invest Ophthalmol Vis Sci. 1988;29(6):876-81.
23. Danser AH, van der Dorpel MA, Deinum J, Derkx FH, Franken AA, Peperkamp E, et al. Renin, prorenin, and immunoreactive renin in vitreous fluid from eyes with and without diabetic retinopathy. J Clin Endocrinol Metab. 1989;68(1):160-7.
24. Ho H, Shi Y, Chua J, Tham YC, Lim SH, Aung T, et al. Association of systemic medication use with intraocular pressure in a multiethnic Asian population: the Singapore Epidemiology of Eye Diseases Study. JAMA Ophthalmol. 2017;135(3):196-202. doi: 10.1001/ jamaophthalmol.2016.5318.
25. Costagliola C, Di Benedetto R, De Caprio L, Verde R, Mastropasqua L. Effect of oral captopril (SQ 14225) on intraocular pressure in man. Eur J Ophthalmol. 1995;5(1):19-25.
26. Costagliola C, Verolino M, De Rosa ML, Laccarino G, Ciancaglini M, Mastropasqua L. Effect of oral losartan potassium on intraocular pressure in normotensive and glaucomatous human subjects. Exp Eye Res. 2000;71(2):167-71.
27. Reitsamer HA, Kiel JW. Relationship between ciliary body blood flow and aqueous production in rabbits. Invest Ophthalmol Vis Sci. 2003;44(9): 3967-71.
28. Lotti VJ, Pawlowski N. Prostaglandins mediate the ocular hypotensive action of the angiotensin converting enzyme inhibitor MK-422 (enalaprilat) in African green monkeys. J Ocul Pharmacol. 1990; 6(1):1-7.
29. Mabuchi F, Aihara M, Mackey MR, Lindsey JD, Weinreb RN. Regional optic nerve damage in experimental mouse glaucoma. Invest Ophthalmol Vis Sci. 2004;45(12):4352- 8.
30. Constad WH, Fiore P, Samson C, Cinotti AA. Use of an angiotensin converting enzyme inhibitor in ocular hypertension and primary open-angle glaucoma. Am J Ophthalmol. 1988;105(6):674-7.
31. Danser AH, Derkx FH, Admiraal PJ, Deinum J, de Jong PT, Schalekamp MA Angiotensin levels in the eye. Invest Ophthalmol Vis Sci. 1994; 35(3):1008-18.
32. Hirooka K, Shiraga F. Potential role for angiotensin-converting enzyme inhibitors in the treatment of glaucoma. Clin Ophthalmol. 2007;1(3):217-23.
33. Osusky R, Nussberger J, Amstutz C, Flammer J, Brunner HR. Individual measurements of angiotensin II concentrations in aqueous humor of the eye. Eur J Ophthalmol. 1994;4(4):228-33.
34. Küchle M, Nguyen NX, Hannappel, Beck W, Ho ST, Naumann GO. [Tyndallometry with the laser flare cell meter and biochemical protein determination in the aqueous humor of eyes with pseudoexfoliation syndrome]. Ophthalmologe. 1994;91(5):578-84. German.
35. Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am J Ophthalmol. 2006;141(5):921-37.
36. Sowka J. Pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Optometry. 2004;75(4):245-50.
Submitted for publication:
November 14, 2017.
Accepted for publication:
March 13, 2018.
Funding: This study was supported by Gazi University BAP Project number 01/2010-29
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose
Approved by the following research ethics committee: Gazi University School of Medicine (# 94/2011)