

Ramazan İlyas Öner1; Ayşe Sevgi Karadağ2
DOI: 10.5935/0004-2749.20180088
ABSTRACT
Purpose: We aimed to evaluate choroidal perfusion changes in obese patients using optical coherence tomography and dynamic contour tonometry, to determine whether these changes are associated with body mass index, and to assess the ocular effects of insulin resistance.
Methods: We retrospectively evaluated the data of 32 obese patients with body mass index >30 kg/m2 and 45 healthy control individuals. Intraocular pressure and ocular pulse amplitude values of the patients were measured using dynamic contour tonometry, and the mean choroidal thickness was measured using enhanced depth imaging optical coherence tomography. Insulin resistance was assessed using the homeostasis model assessment-estimated insulin resistance index.
Results: The mean choroidal thickness (294.30 ± 60.87 µm) and ocular pulse amplitude (2.10 ± 0.74) were lower, whereas the mean intraocular pressure (16.61 ± 2.35 mmHg) was higher in obese patients than in controls. There was a significant negative correlation between body mass index and ocular pulse amplitude (r=-0.274; p=0.029) and an insignificant negative correlation between mean choroidal thickness, intraocular pressure, and body mass index. There was an insignificant negative correlation between homeostasis model assessment-estimated insulin resistance index, mean choroidal thickness, and intraocular pressure and significant negative correlation between homeostasis model assessment-estimated insulin resistance index and ocular pulse amplitude (r=-0.317; p=0.022).
Conclusion: We found reduced mean choroidal thickness and ocular pulse amplitude and increased mean intraocular pressure in obese patients. These changes indicated a decrease in choroidal perfusion and ocular blood flow. It may be possible to detect ocular blood flow changes in obese patients through noninvasive assessment using the choroid. The negative correlation between insulin resistance and ocular pulse amplitude may be associated with intracellular fat accumulation in obese patients.
Keywords: Obesity; Insulin resistance; Choroidal perfusion; Dynamic contour tonometry; Optical coherence tomography
RESUMO
Objetivo: Avaliar as alterações da perfusão coroidiana em pacientes obesos utilizando tomografia de coerência óptica e a tonometria de contorno dinâmico, para determinar se essas alterações estão associadas ao índice de massa corporal e avaliar os efeitos oculares da resistência à insulina.
Métodos: Foram avaliados, retrospectivamente, os dados de 32 pacientes obesos, com índice de massa corporal >30 kg/m2, e 45 controles saudáveis. Os valores de pressão intraocular e da amplitude de pulso ocular dos pacientes foram medidos por meio de tonometria de contorno dinâmico e a espessura média da coroide foi medida por tomografia de coerência óptica com profundidade de imagem aprimorada. A resistência à insulina foi avaliada usando o índice de estimativa da resistência à insulina pelo modelo de homeostase.
Resultados: A espessura média da coroideia (294,30 ± 60,87 µm) e a amplitude de pulso ocular (2,10 ± 0,74) foram menores, enquanto a pressão intraocular média (16,61 ± 2,35 mmHg) foi maior nos obesos do que nos controles. Houve uma correlação negativa significativa entre o índice de massa corporal e a amplitude de pulso ocular (r=-0,274; p=0,029) e uma correlação negativa insignificante entre a espessura média da coroide, a pressão intraocular e o índice de massa corporal. Houve uma correlação negativa insignificante entre a avaliação do modelo de homeostase - estimativa do índice de resistência à insulina, espessura média da coróide e pressão intraocular e correlação negativa significativa entre o modelo de avaliação de homeostase - o índice de resistência à insulina estimado e a amplitude de pulso ocular (r=-0,317; p=0,022).
Conclusão: Encontramos redução da espessura média da coroide e da amplitude de pulso ocular e aumento da pressão intraocular em pacientes obesos. Essas alterações indicaram uma diminuição na perfusão coroidal e no fluxo sanguíneo ocular. Pode ser possível detectar alterações no fluxo sanguíneo ocular em pacientes obesos por meio de avaliação não invasiva usando a coróide. A correlação negativa entre a resistência à insulina e a amplitude de pulso ocular pode estar associada ao acúmulo de gordura intracelular em pacientes obesos.
Descritores: Obesidade; Resistência a insulina; Perfusão de coroide; Tonometria de contorno dinâmico; Tomografia de coerência óptica
INTRODUCTION
Obesity, which is characterized by fat accumulation, is often associated with fatty tissue dysfunction(1). Inflammatory, endocrine, hormonal (leptin and ghrelin levels), and metabolic factors induce morphological and functional changes in fatty tissue, resulting in obesity(2).
Obesity is one of the important risk factors for many disorders, such as hypertension, diabetes mellitus, dyslipidemia, stroke, and cardiovascular diseases(3). It affects the vascular system at the morphological and functional levels(4) and may lead to endothelial dysfunction and vascular damage of the eye, resulting in impaired blood flow and vascular structure(5). Although the effects of obesity on the eyes are not completely identified, it is known to cause microvascular changes in the retinal and choroidal veins; it has been associated with several diseases, such as diabetic retinopathy, glaucoma, cataract, and age-related macular degeneration(6).
The choroid can be evaluated using optical coherence tomography (OCT) or dynamic contour tonometry (DCT) to detect the changes caused by obesity in the choroidal vascular bed. While choroidal thickness (CT) can be directy measured using OCT, choroidal perfusion and intraocular blood flow can be indirectly assessed using ocular pulse amplitude (OPA)(7). OPA provides an indirect measure of choroidal pulsatile blood flow by reflecting the difference in intraocular pressure (IOP) between the systole and diastole during the cardiac cycle, which is noninvasively measured using DCT(8).
In our study, we aimed to evaluate the changes in choroidal perfusion using both OCT and DCT in obese patients, demonstrate the association of these changes with body mass index (BMI), and assess the ocular effects of insulin resistance (IR).
METHODS
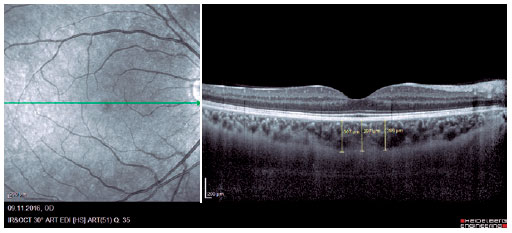
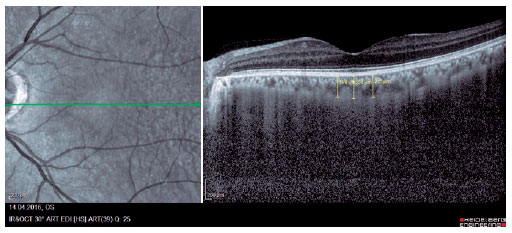
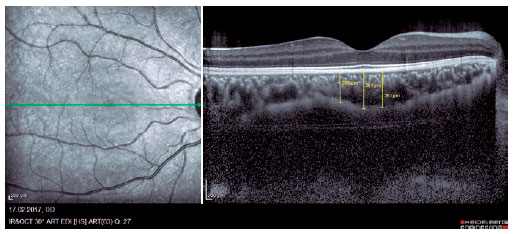
We retrospectively evaluated 32 obese patients with BMI of >30 kg/m2 who presented at the Internal Medicine Polyclinic due to obesity and who were also evaluated at the Ocular Diseases Polyclinic between January 2016 and June 2017 and 45 healthy control individuals. Eye IOP and OPA values of patients measured using Pascal DCT (Ziemer Group, Switzerland) and CT measured using enhanced depth imaging (centralized fovea for choroildal imaging) OCT (85,000 Hz OCT2 Next Generation SPECTRALIS OCT, Heidelberg Engineering) were assessed. These values were measured by the same physician and at the same time of the day. Only the most reliable measurements with scores 1 and 2 were included in the study. CT was manually measured by a single ophthalmologist based on the external boundary of retinal pigment epithelium and that of the choroidal vascular bed. It was measured three times at the subfoveal, nasal, and temporal regions at a distance of 500 µm from the macula, and the average of these three measurements was used for analyses (Figures 1-3). IR was assessed using the homeostasis model assessment-estimated IR (HOMA-IR) index(9), which was calculated as follows: HOMA-IR=[fasting plasma insulin (mIU/mL) × fasting plasma glucose (mmol/L)]/ 22.5(10). HOMA-IR index of 2.5 was set as the cutoff value(11). Patients with ocular tension and corneal disease, those with nonobesity systemic diseases, those who underwent eye surgery, and those who underwent interventions such as laser therapy and intravitreal injections were excluded from the study. This study was approved by the Ethics Committee of the Adiyaman University (#2017/7-19).
Statistical methods
We used the Kolmogorov-Smirnov test to evaluate the assumption of normality of the data. The data were presented as the frequency (percentages), mean ± standard deviation (SD), median, and range. Between-group differences were evaluated using the t-test, Mann-Whitney U test, and chi-square test; the generalized estimating equation analysis was used to compare groups while considering the correlation between the eyes in one subject. A p-value of <0.05 was considered as statistically significant. All statistical analyses were performed using the SPSS software (Version 21.0, Microsoft, Chicago, IL, USA). The Spearman’s correlation test was used to analyze the correlation between the variables.
RESULTS
We evaluated 32 obese patients [24 female (75%) and 8 male (25%)], with a mean age of 41.90 ± 11.88 years. The mean BMI of the patients was 36.04 ± 5.34 kg/m2 (range, 30.00-54.70 kg/m2).
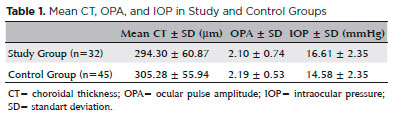
The mean CT (294.30 ± 0.87 µm) and OPA (2.10 ± 0.74) values were lower and the mean IOP (16.61 ± 2.35 mmHg) was higher in obese patients than in healthy controls. The mean CT, OPA, and IOP values of all the subjects are shown in table 1.
A significant negative correlation was found between BMI and OPA in obese patients (r=-0.274; p=0.029), whereas negative but no significant correlation was found between mean CT, IOP, and BMI (Table 2).
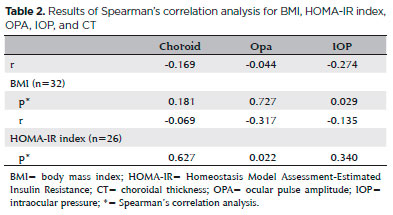
In addition, we found a positive correlation between CT and IOP and negative correlation between CT and OPA, both of which were not statistically significant. However, there was a significant positive correlation between OPA and IOP (r=0.492; p=0.000).
Furthermore, there was a significant negative correlation between HOMA-IR index and OPA (r=-0.317; p=0.022; n=26), whereas the correlation between HOMA-IR index and CT and that between HOMA-IR index and IOP were negative but not statistically significant. There was a significantly positive correlation between BMI and HOMA-IR index (r=0.288; p=0.038) (Table 2).
DISCUSSION
CT has a predictive and prognostic value for localized diseases, such as diabetic retinopathy, and systemic diseases, such as hypertension and rheumatoid arthritis(12,13). Several studies have hypothesized that microvascular changes in the choroid in obese patients are noninvasive indicators for secondary vascular changes, such as cardiovascular diseases(14,15). Obesity is also considered a risk factor for retinopathy(16).
However, there are limited number of studies on CT, OPA, and IOP in obese individuals, with conflicting results(15,17). Furthermore, the assessment of OPA and IOP in obese individuals, regardless of CT, has been shown as an important limiting factor in one of the studies(18). Therefore, we assessed CT using OCT and examined the association between OPA and IOP using DCT.
An important finding of this study was the decreased mean CT in obese patients compared with that in controls. Erşan et al. found significantly decreased macular and subfoveal CT in obese children(19). Yilmaz et al. reported a negative correlation between BMI and CT(15). Dogan et al. also reported an increase in postoperative subfoveal CT in morbidly obese individuals at 3- and 6-month follow-ups(20). However, contradictory to the above findings, Yumuşak et al. found a positive correlation between BMI and CT in females and suggested that ocular circulation was the main parameter affected due to obesity(21). Hyperinsulinemia and high blood pressure have been reported to be responsible for the microvascular changes in obese females(22).
Bulus et al. reported a positive correlation between BMI and subfoveal CT in obese children, which may be attributed to increased leptin levels and obesity-associated inflammatory factors in obese individuals(17). Additionally, the diffrences in the outcomes regarding CT may have resulted due to differences in the populations studied.
Another important finding in our study was a statistically significant increase in IOP in obese patients, which is similar to the findings of a previous study(23). Obesity is thought to affect IOP due to the presence of excessive intraorbital adipose tissue, increased episcleral venous pressure, and disrupted aqueous outflow(24), resulting from the reduction in nitric oxide levels and imbalance between vasoconstrictor and vasodilator levels, such as increased endothelin-1 and angiotensin-II levels(25). Also, increased oxidative stress due to hyperleptinemia in obese patients may trigger the pathological changes resulting in increased IOP(26). Therefore, obesity is considered an independent risk factor for increase in IOP.
Our study is the only study in which DCT was used for optical assessment in obese patients. As it is the only study we have identified in which DCT is used for assessment in obese patients, Karadağ et al. reported that IOP increased in obese patients, but this increase was not significant between IOP and BMI(27).
Furthermore, we also found that there was a significant decrease in OPA with an increase in BMI, which was consistent with the findings of Karadağ et al.(27). This decrease in OPA in obese patients may be attributed to the presence of increased periorbital adipose tissue, which hampers choroidal perfusion and ocular blood flow.
We found a negative correlation between BMI and CT, OPA, and IOP; insignificant negative correlation between HOMA-IR index, which is used as an indicator for IR, and CT and IOP; and significant negative correlation between HOMA-IR index and OPA. In obesity, IR develops due to different mechanisms, such as impaired insulin signaling and glucose homeostasis alteration, caused by intracellular fat accumulation(28). Increased body weight causes a reduction in insulin sensitivity and IR through various mechanisms, such as suppression of adiponectin production, downregulation of insulin receptor expression, and reduction of tyrosine kinase activity in adipocytes and skeletal muscle(29). Thus, IR may cause a similar effect on choroidal perfusion because it is significantly correlated with body fat(30).
In our literature survey, we found that although there are studies on OCT performed for obese patients, there was only one study wherein DCT was used(30) and no studies wherein OCT and DCT were used together. Therefore, our study is the first to use both OCT and DCT for ocular evaluation in obese patients.
Taken together, we found that there was a decrease in CT and OPA and an increase in IOP in obese patients. These changes indicated reduced choroidal perfusion and ocular blood flow. An increase in IOP implied increased sensitivity to some ocular diseases, such as glaucomatous diseases, in obese individuals. Early development of vascular anomalies over the course of obesity suggested that it is possible to noninvasively determine the status of ocular blood flow using choroidal assessment. Because central or intra-abdominal obesity is associated with IR, the negative correlation between IR and OPA in obese patients reflected intracellular fat accumulation. It is necessary to conduct further studies to determine whether CT changes are reversible with weight loss and whether weight loss reduces the risk of ophthalmic diseases(6).
REFERENCES
1. Ahmadian M, Wang Y, Sul HS. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42(5):555-9.
2. Yudkin JS, Eringa E, Stehouwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365(9473):1817-20.
3. Grundy SM. Metabolic complications of obesity. Endocrine. 2000; 13(2):155-65.
4. Ciccone MM, Miniello V, Marchioli R, Scicchitano P, Cortese F, Palumbo V,et al. Morphological and functional vascular changes induced by childhood obesity. Eur J Cardiovasc Prev Rehabil. 2011; 18(6):831-5.
5. Grieshaber MC, Flammer J. Blood flow in glaucoma. Curr Opin Ophthalmol. 2005;16(2):79-83.
6. Cheung N, Wong TY. Obesity and eye diseases. Surv Ophthalmol. 2007;52(2):180-95.
7. Pekel G, Acer S, Yağci R, Özdemir S, Kaya H, Hiraali MC,et al. Relationship between subfoveal choroidal thickness, ocular pulse amplitude, and intraocular pressure in healthy subjects. J Glaucoma. 2016;25(7):613-7.
8. Punjabi OS, Kniestedt C, Stamper RL, Lin SC. Dynamic contour tonometry: principle and use. Clin Exp Ophthalmol. 2006;34(9): 837-40.
9. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-9.
10. Qu HQ, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS One. 2011;6(6):e21041.
11. Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500-3.
12. Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 2012; 32(3):563-8.
13. Duru N, Altinkaynak H, Erten Ş, Can ME, Duru Z, Uğurlu FG, et al. Thinning of choroidal thickness in patients with rheumatoid arthritis unrelated to disease activity. Ocul Immunol Inflamm. 2015;31:1-8.
14. Boillot A, Zoungas S, Mitchell P, Klein R, Klein B, Ikram MK,et al. META-EYE Study Group. Obesity and the microvasculature: a systematic review and meta-analysis. PLoS One. 2013;8(2):e52708.
15. Yilmaz I, Ozkaya A, Kocamaz M, Ahmet S, Ozkaya HM, Yasa D, et al. Correlation Of choroidal thickness and body mass index. Retina. 2015;35(10):2085-90.
16. Van Leiden HA, Dekker JM, Moll AC, Nijpels G, Heine RJ, Bouter LM, et al. Blood pressure, lipids, and obesity are associated with retinopathy: The Hoorn Study. Diabetes Care. 2002;25(8):1320-5.
17. Bulus AD, Can ME, Baytaroglu A, Can GD, Cakmak HB, Andiran N. Choroidal Thickness in Childhood Obesity. Ophthalmic Surg Lasers Imaging Retina. 2017;48(1):10-7.
18. Son J, Koh H, Son J. The association between intraocular pressure and different combination of metabolic syndrome components. BMC Ophthalmol. 2016;16(1):76.
19. Erşan I, Battal F, Aylanç H, Kara S, Arikan S, Tekin M, et al. Noninvasive assessment of the retina and the choroid using enhanced-depth imaging optical coherence tomography shows microvascular impairments in childhood obesity. J AAPOS. 2016;20(1):58-62.
20. Dogan B, Dogan U, Erol MK, Habibi M, Bulbuller N. Optical coherence tomography parameters in morbidly obese patients who underwent laparoscopic sleeve gastrectomy. J Ophthalmol. 2016;2016:5302368.
21. Yumusak E, Ornek K, Durmaz SA, Cifci A, Guler HA, Bacanli Z. Choroidal thickness in obese women. BMC Ophthalmol. 2016; 16(1):48.
22. de Jongh RT, Serné EH, IJzerman RG, de Vries G, Stehouwer CD. Impaired microvascular function in obesity: implications for obesity-associated microangiopathy, hypertension, and insulin resistance. Circulation. 2004;109(21):2529-35.
23. George GO. Relationship between body mass index, intraocular pressure, blood pressure and age in Nigerian population. J Clin Exp Ophthalmol. 2015;6(4):4-8.
24. Stojanov O, Stokić E, Sveljo O, Naumović N. The influence of retrobulbar adipose tissue volume upon intraocular pressure in obesity. Vojnosanit Pregl. 2013;70(5):469-76.
25. Stapleton PA, James ME, Goodwill AG, Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15(2):79-89.
26. Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123(4):458-63.
27. Karadag R, Arslanyilmaz Z, Aydin B, Hepsen IF. Effects of body mass index on intraocular pressure and ocular pulse amplitude. Int J Ophthalmol. 2012;5(5):605-8.
28. Rimm EB, Stampfer MJ, Giovannucci E, Ascherio A, Spiegelman D, Colditz GA, et al. Body size and fat distribution as predictors of coronary heart disease among middle-aged and older US men. Am J Epidemiol. 1995;141(12):1117-27.
29. Olefsky JM. The insulin receptor: its role in insulin resistance of obesity and diabetes. Diabetes. 1976;25(12):1154-62.
30. Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Vessby B, Basu S, et al. Relation of body mass index and insulin resistance to cardiovascular risk factors, inflammatory factors, and oxidative stress during adolescence. Circulation. 2005;111(15):1985-91.
Submitted for publication:
January 22, 2018.
Accepted for publication:
March 13, 2018.
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose
Approved by the following research ethics committee: Adiyaman University (#2017/7-19)