

Alper Mete1, Sabit Kimyon1; Oguzhan Saygılı1; Yusuf Koçluk2; Iffet Yarımağa Kaçarlar1; Kıvanç Güngör1; Mithat Temizer3
DOI: 10.5935/0004-2749.20180060
ABSTRACT
Purpose: Scleral fixation surgery is a pivotal procedure that depends on the availability of robust and innovative surgical fixation methods. There continues to be a need for innovation in suture fixation techniques, particularly for intraocular lens implantation.
Methods: We conceived and designed a “knot ball” scleral fixation technique for suture burial in a retrospective sample of 108 patients with primary (n=40) or secondary (n=68) scleral-fixated intraocular lens. Importantly, our technique did not require additional scleral flap or tunnel procedures. We evaluated pre- and postoperative best-corrected visual aquity (BCVA) and postoperative complications. All data were analyzed and compared between groups.
Results: The preoperative mean BCVA improved significantly in both groups using the “knot ball” fixation technique (p<0.01). The extent of the improvement in the best-corrected visual acuity and late complications one month post-surgery were not significantly different between the groups (p>0.05). These clinical outcomes were consistent with those described in the ophthalmology literature.
Conclusion: A “knot ball” scleral fixation technique is reported; to the best of our knowledge, this is the first report of such a technique, which offers a less invasive and simplified surgical procedure for transscleral fixation of scleral-fixated intraocular lenses. Moreover, the technique appears to display similar effectiveness and safety compared with existing scleral fixation techniques. We suggest that the “knot ball” technique warrants further research focus and clinical evaluation in future studies.
Keywords: Sclera/surgery; Lens implantation; Intraocular; Aphakia; Sutures techniques
RESUMO
Objetivo: A cirurgia de fixação escleral é um procedimento fundamental que depende da disponibilidade de métodos robustos e inovadores de fixação cirúrgica. No entanto, existe uma necessidade de inovação nas técnicas de fixação de sutura, particularmente para a implantação de lentes intraoculares.
Métodos: Concebemos e desenhamos uma técnica de fixação escleral utilizando um “nó esférico” para o encerramento da sutura em uma amostra retrospectiva de 108 pacientes com lente intraocular de fixação escleral (SF-IOL) primária (n=40) e secundária (n=68). Importante considerar que nossa técnica não exigiu procedimentos adicionais de aleta escleral ou de túnel. Observamos a melhor acuidade visual corrigida (MAVC) pré e pós-operatória e as complicações pós-operatórias. Todos os dados foram analisados entre os grupos.
Resultados: A melhor acuidade visual corrigida média pré-operatória (logMAR) melhorou significativamente em ambos os grupos com a utilização da técnica de fixação do nó esférico (p<0,01). A extensão da melhora melhor acuidade visual corrigida e as complicações tardias, um mês após a cirurgia, não foram significativamente diferentes entre os grupos (p>0,05). Esses resultados clínicos foram, em geral, comparáveis aos publicados na literatura de oftalmologia.
Conclusão: Até onde sabemos, a técnica de fixação escleral de “nó esférico” é relatada pela primeira vez na literatura e representa um procedimento cirúrgico promissor, menos invasivo e simplificado para a fixação transescleral de SF-IOLs. Além disso, a técnica parece exibir eficácia e segurança comparáveis às técnicas de fixação escleral existentes. Sugerimos que a técnica do nó esférico receba mais atenção e avaliações clínicas no futuro.
Descritores: Esclera/cirurgia; Implante de lente intraocular; Afacia; Técnicas de sutura
INTRODUCTION
Innovation in ophthalmic surgery is an area of intense research in which new technologies or procedural improvements might offer the promise of improved clinical outcomes. In this context, scleral fixation surgery is a crucial procedure which depends on the availability of robust scleral fixation methods. There is a particular need for new approaches in suture fixation techniques for intraocular lens implantation to make the procedures less invasive.
In the absence of sufficient capsular support, scleral-fixated (SF) posterior chamber intraocular lenses (IOLs), anterior chamber IOLs (ACIOLs), iris-claw IOLs, or iris-sutured posterior chamber IOLs are used for surgical treatment of aphakia(1). Previous studies have suggested that transscleral fixation of posterior chamber IOLs has significant advantages over other implantation techniques in the context of prevention of various complications, including corneal endothelial damage, pupillary block glaucoma, hyphemia, uveitis, iris damage, IOL dislocation, and pseudophakodonesis(2-4). Transscleral fixation of posterior chamber IOLs can be performed using suture or sutureless techniques(5). Suture techniques need to cover, bury, or rotate suture knots to prevent suture-related complications(2,3).
Numerous techniques have been described for avoiding suture exposure and suture-related complications such as creating a scleral flap for suture knots(6), suturing into a scleral groove(7) or pocket(8), burying the suture ends into a scleral tunnel(9), direct suture burial without a flap, tunnel, incision, or groove(10), covering the suture knots with fascia lata, tenon’s capsule(11), cornea, or scleral autograft(12), knotless Z-suture techniques(13), sutureless techniques using fibrin glue, and trocar- or needle-assisted techniques(14-16). The ideal transscleral fixation technique should be simple, quick, minimally invasive, safe, effective, and offer easy management of potential surgical complications.
Here, we present a simple, quick, minimally invasive, and effective alternative technique, a new “knot ball scleral fixation technique” for burying ball-shaped suture knots directly into the sclera for transscleral fixation of SFIOL; we compared the visual outcomes and complications in primary and secondary implantations using our technique.
METHODS
Subjects
We retrospectively analyzed the medical records of 108 patients (65 males, 43 females; 108 eyes) who underwent SFIOL implantation surgery with our new “knot ball” scleral fixation technique without creating a scleral flap, groove, or tunnel. The study and analysis were conducted at the Gaziantep University Hospital between March 2014 and October 2015. This study was approved by the institutional research ethics board and adhered to the tenets of the Declaration of Helsinki. All patients had a complete preoperative evaluation, including best-corrected visual acuity (BCVA) and IOP measurements, and slit-lamp and fundus examinations. Subjects were informed about the procedure, potential complications, and possible need for secondary surgery
The indications for SFIOL implantation were extensive capsular deficiency, crystalline lens subluxation preventing safe and stable IOL implantation in the bag or sulcus, corneal decompensation due to ACIOL, IOL subluxation due to trauma, or pseudoexfoliation syndrome. The decision regarding primary or secondary implantation was made based on the preference of the surgeon depending on the type of anesthesia, comfort, and general medical condition of the patient. All surgeries were performed using the new “knot ball” suture fixation technique by two experienced surgeons (O.S. and A.M.). Patients who were followed up for at least nine months were included in the study. Intraoperative and postoperative complications and visual outcomes were noted. All data were compared between groups.
The “knot ball” scleral fixation technique
Figure 1 and 2 show the steps involved in SFIOL implantation using our technique. In brief, the procedure comprised the following steps and details. Preoperatively, IOL power was calculated using the Sanders-Retzlaff-Kraff II formula. Pupils were dilated with 1% tropicamide, 2.5% phenylephrine hydrochloride, and 1% cyclopentolate. The periocular area and eye were cleaned with 5% povidone iodine. In the primary implantation group, the procedure was initiated as a standard phacoemulsification or extracapsular cataract extraction surgery with a corneal incision at the 12 o’clock meridian. If there were any posterior capsule or lens zonule complications during cataract surgery, corneal incision was enlarged to approximately 6.5 to 7.0 mm, and lens fragments were explanted. In the secondary implantation group (aphakic or pseudophakic), a 6.5 to 7.0 mm clear corneal incision was created at the 12 o’clock meridian, and any subluxated IOL or ACIOL was explanted in pseudophakic cases. Surgeries in both groups were continued as following. Limbus-parallel conjunctival peritomies (~2 mm) were created at 3 o’clock and 9 o’clock meridians 2 mm behind the limbus. The sclera was not cauterized and only cotton buds were used for bleeding control in all patients. Next, triamcinolone acetonide (0.5 cc) was injected into the anterior chamber to stain the vitreous for direct visualization and anterior vitrectomy was performed using a 20-gauge vitrectomy cutter. The 10-0 closed-loop polypropylene suture (PC-9, ALCON, Fort Worth, Texas or MANI Inc., Togichi, Japan) ends were attached to the haptics of a PSV 651 single piece polymethylmethacrylate SFIOL (FREEDOM, Tamil Nadu, India). Subsequently, half of the curved needle was passed directly through the sclera approximately 2 mm behind the limbus at the 3 o’clock meridian. A micro hole was made at the sclera-curved needle junction using the tip of a 20-gauge microvitreoretinal (MVR) blade under a high magnification surgical microscope (Figure 1F-H, Figure 3A). Next, the same procedure was performed on the opposite side (i.e., 9 o’clock meridian).
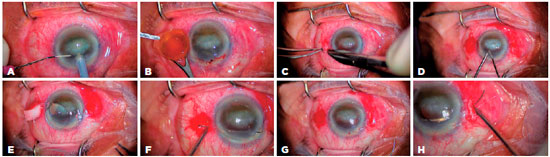
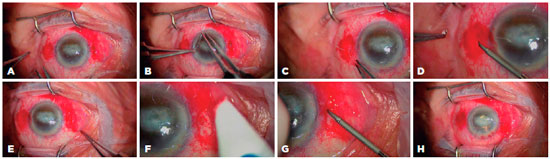
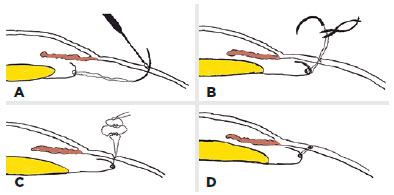
While implanting each haptic of the SFIOL into the posterior chamber, the curved needle was gently pulled. After implantation, the closed-loop suture was cut approximately 1.5 to 2.0 cm away from the needle-perforated scleral point so that two fibers were formed from the closed-loop suture; these fibers were separated for suturing (Figure 3B). The knot was tied in a 3-2-1 fashion using both suture fibers of the 10-0 closed-loop polypropylene suture to create a ball-shaped knot for scleral fixation (Figure 3C). We buried the knot ball directly into the steep scleral tunnel which was formed by the curved-needle and the tip of a 20-gauge MVR blade without any additional procedures (Figure 3D). The IOL manipulator was used for achieving centralization of the SFIOL and checking the burial of the ball-shaped suture knot. The corneal incision was closed using 10-0 monofilament nylon sutures and conjunctival peritomies were closed using 8-0 absorbable sutures. Finally, an antibiotic was delivered into the anterior chamber.
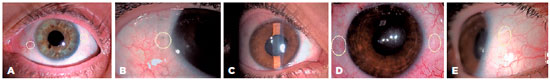
Postoperatively, topical antibiotic and steroid drops were administered six times a day for four weeks and anti-glaucomatous drops were administered when required. Follow-up examinations were performed on the first day, the first week, and then monthly after the surgical procedure. Figure 4 shows the postoperative status of two patients. Patient demographics, preoperative and postoperative BCVAs, follow-up periods, preoperative lens status of eyes, and postoperative complications were noted. The BCVAs were measured in decimal units and converted into logarithms of the minimal angle of resolution (logMAR) units for analysis. Visual acuities of hand movement and light perception were converted into equivalents of 1.7 and 1.8 logMAR units, respectively(17,18).
Statistical analyses
The software SPSS 16.0 for Windows (SPSS Inc, Chicago, IL, USA) was used to analyze outcomes and changes in the study endpoints. Patient demographics, follow-up periods, and postoperative complications were compared between primary and secondary SFIOL implanted groups using Chi-squared tests. Preoperative and postoperative BCVAs were statistically analyzed using paired samples t-tests. A p-value less than 0.05 was considered significant.
RESULTS
We performed the “knot ball” technique in 40 eyes (37.1%) with primary implantation (the primary group) and in 68 eyes (62.9%) with secondary implantation (the secondary group). Patient characteristics, length of the follow-up period, and preoperative and postoperative BCVAs are summarized in table 1.
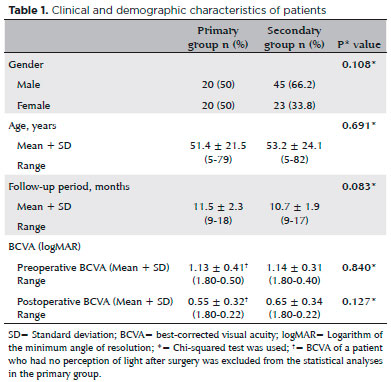
There were no statistically significant differences between the study groups in age, gender, duration of follow-up, preoperative BCVA, or postoperative BCVA (p>0.05 for all analyses). Indications for SFIOL implantation and preoperative lens status of cases are summarized in table 2.
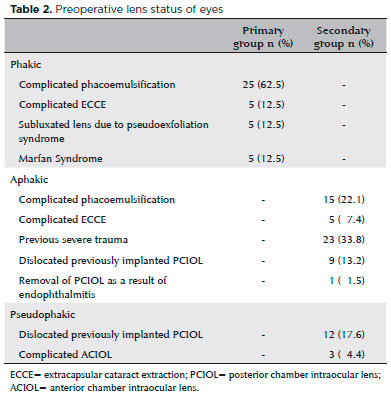
The preoperative mean BCVAs (logMAR) were 1.13 ± 0.41 in the primary group and 1.14 ± 0.31 in the secondary group. Postoperative mean BCVAs were 0.55 ± 0.32 in the primary group and 0.65 ± 0.34 in the secondary group. There were no statistically significant differences in preoperative and postoperative mean BCVAs between the groups (p=0.840 and p=0.127, respectively). In the primary group, BCVA improved in 33 (82.5%) eyes and remained unchanged in three (7.5%), while in the secondary group, BCVA improved in 60 (88.2%) eyes and remained unchanged in six (8.8%). The postoperative BCVA improved statistically significantly compared with the preoperative BCVA in both groups (p<0.01 for both groups). Deterioration of vision occurred in four (10%) eyes (suprachoroidal hemorrhage, n=1; retinal detachment, n=1; cystoid macular edema, n=1; endophthalmia, n=1) in the primary group and in two (2.9%) eyes (cystoid macular edema, n=1; IOL dislocation, n=1) in the secondary group.
Postoperative complications are summarized in table 3. During the early postoperative period, complications occurred in 20 (50%) and 13 (19.1%) eyes in the primary and secondary groups, respectively. There was a statistically significant difference between groups in incidence of early complications (p=0.02). Transient IOP elevation and transient corneal edema were the most common complications in both groups during the early period. These patients were treated successfully using anti-glaucomatous and steroid drops in both groups. In the primary group, four (10%) eyes required secondary surgical intervention due to early period complications (IOL dislocation, n=2; suprachoroidal hemorrhage, n=1; endophthalmitis, n=1). Eyes with IOL dislocation were treated successfully and an increase in visual acuity was attained after the secondary intervention. The BCVA deteriorated only in two patients. One patient (with fungal endophthalmitis) had no perception of light, and another patient (with suprachoroidal hemorrhage) had light perception following secondary surgical interventions. Endophthalmitis occurred two days after surgery, indicating that it may be related to intraoperative contamination. In the secondary group, secondary surgical intervention was not required for any patient.
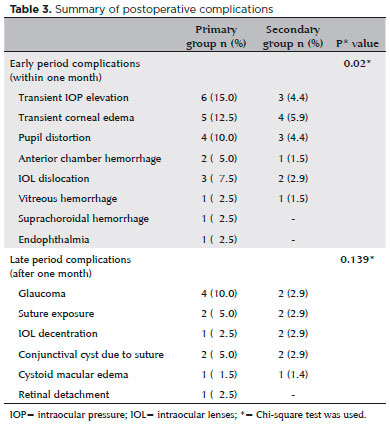
There was no statistically significant difference between groups in the incidence of late postoperative period complications (p=0.139). Late postoperative period complications were observed in 11 (27.5%) and nine (13.2%) eyes in the primary and secondary groups, respectively. Glaucoma (four eyes) was the most common late postoperative period complication in the primary group. These patients were successfully treated using anti-glaucomatous drops. In the secondary group, one of the glaucoma cases was treated with anti-glaucomatous drops, while the other one was treated using surgical intervention. We observed IOL decentration in one eye in the primary group and in two eyes in the secondary group. We performed secondary surgical intervention in these three eyes due to deteriorated vision. Suture exposure occurred in two (5%) eyes in the primary group and in two (2.9%) eyes in the secondary group. We treated all these cases using cauterization(14). Retinal detachment occurred in one patient (2.5%) in the primary group who required secondary surgical intervention. Overall, eight (20%) eyes in the primary group and five (7.4%) eyes in the secondary group required secondary surgical interventions due to postoperative complications. Our outcomes showed that there was no significant difference between groups in the requirement of secondary surgical intervention (p=0.159). We observed cystoid macular edema in one patient in the primary group (1.5%) and in one patient (1.4%) in the secondary group, with spontaneous resolution occurring in both cases.
DISCUSSION
There have been several methods of transscleral fixation of posterior chamber intraocular lenses (PCIOLs). Among these, the triangular scleral flap is one of the most commonly used techniques worldwide. However, this technique extends surgery duration, requires more scleral bleeding control during surgery, and atrophy in scleral flaps may occur over time(20). Hoffman et al.(8) described a minimally invasive technique without conjunctival dissection, in which scleral pockets were created through peripheral clear corneal incisions. However, this technique requires passing twice through the sclera for each haptic fixation and corneal incision. Additionally, Hoffman’s technique presents a potential risk of astigmatism compared with other techniques. Some authors have reported the use of scleral grooves instead of scleral flaps for transscleral fixation(21-23). The scleral groove technique and its modifications reduce surgery duration and the rate of suture-related complications. However, the scleral groove technique and its modifications require at least two passes through the sclera, additional incisions for scleral grooves, additional sutures for grooves, and risk of vitreous hemorrhage due to scleral passes remains. Kir et al.(9) have described a scleral tunnel technique for burying suture ends into the scleral tunnel without creating a scleral flap pocket or grooves. However, this technique requires additional suturing procedures for free suture ends. Some surgeons have described graft or tissue-dependent techniques such as covering the suture knots with fascia lata, tenon’s capsule(11), or corneal or scleral autograft(12); however, these techniques present disadvantages, including the requirement of additional surgical procedures and prolonged surgery duration. Baykara(10) has described a technique for suture knot burying into the sclera without flaps, tunnels, or grooves. The new technique we developed and described in this report, the “knot ball” scleral fixation approach, displays some similarities to the Baykara’s direct suture burial technique. Yet, there are also important distinctions in that we pass only once through the sclera for each haptic and we create a ball-shaped suture knot ball, which is then embedded into the steep scleral tunnel formed by the passage of the curved suture needle and the tip of a 20-gauge MVR blade, without any additional procedures.
Some surgeons have described knotless techniques for transscleral fixation of PCIOLs. Kongsap(1) described knotless zigzag scleral fixation for PCIOLs. This technique is a safe and viable option that reduces surgery duration and postoperative suture-related complications. However, Kongsap’s technique requires multiple passes through the sclera for each haptic. Sutureless techniques for scleral fixation of PCIOLs have become increasingly popular(14-16,24,25). Gabor and Pavlidis(24) introduced sutureless transscleral fixation of a three-piece PCIOL. Agarwal et al.(14) presented a fibrin glue-assisted technique in which the intrascleral haptic of a PCIOL is fixed into a scleral tunnel. Totan and Karadağ(15) presented a trocar-assisted sutureless transscleral fixation technique, and Yamane et al.(16) presented a 27-gauge needle-assisted transscleral fixation technique. However, these sutureless techniques have the potential risk of haptic exposure and require scleral flaps, tunnels, or fibrin glue. Ohta et al.(25) described a sutureless Y-fixation technique that was used to fix a PCIOL without large lamellar scleral flaps or fibrin glue. This technique eliminates the risk of haptic exposure without creating large scleral flaps or using fibrin glue. However, scleral incisions and mini flaps are needed for this technique. We thought that transscleral suture fixation techniques are safer than sutureless techniques because current commercially available IOLs are specially designed for transscleral suture fixation.
Our outcomes showed that the rate of early and late postoperative complications and postoperative visual outcomes were similar to those in previous reports(6,26). Lee et al.(26) performed transscleral PCIOL fixation in 55 patients using an ab externo sulcus fixation technique, which was described by Lewis in 1991(27). They reported that early-period complications occurred in 25 (83.3%) eyes in the primary group and in 16 (64%) eyes in the secondary group. They showed that early-period complications were statistically significantly higher in the primary group. Late-period postoperative complications occurred in 21 (70.0%) eyes in the primary group and in 13 (52.0%) eyes in the secondary group, with no significant differences between the groups regarding late-period complications. Yalniz-Akkaya et al.(6) performed transscleral SFIOL fixation using a triangular scleral flap technique; they reported that early-period postoperative complications occurred in 13 (35.1%) eyes in the primary group and in 17 (28.8%) eyes in the secondary group. The authors showed that late-period postoperative complications occurred in 30 (81.0%) eyes in the primary group and in 40 (67.9%) eyes in the secondary group. They reported no statistically significant differences between groups in early or late postoperative complications. In our study, early postoperative complications occurred in 20 (50%) eyes in the primary group and in 13 (19.1%) eyes in the secondary group. We showed a statistically significant difference between the groups regarding incidence of early postoperative complications (p=0.02). Early postoperative complications mostly occurred in the primary group, but late postoperative complication rates were not significantly different between the primary and secondary implantation groups. Moreover, there was no significant difference in visual outcomes between the groups. We suggest that the decision regarding primary or secondary implantation should be made based on patient characteristics and experience of the surgeon.
Lee et al.(26) showed that the mean postoperative logMAR BCVA was 0.50 and 0.36 in the primary and secondary groups, respectively. Akkaya et al.(6) reported that the mean postoperative logMAR BCVA in the primary group was 0.50 and that in the secondary group was 0.5. In both studies, the authors showed no statistically significant difference between the groups in terms of visual outcomes. Similarly, there was no significant difference between the groups in mean postoperative logMAR BCVA in our study.
The “knot ball” scleral-fixation technique we describe eliminates risky maneuvers such as creating scleral flaps, tunnels, grooves, or two or more scleral perforations for fixation of each haptic. Moreover, this technique provides sufficient IOL centration, minimizes postoperative complications, and reduces surgery time and scleral hemorrhage during surgery. Our technique is especially helpful in treatment of IOL dislocations because it is less invasive and is easier than other methods. The dislocated IOL haptic can easily be refixated with only one pass through the sclera. However, our modified technique involves some critical steps. First, the burial extent of the first three loop-like sutures of the knot is the first critical step, which needs experience; if the suture knot is excessively buried, this may cause IOL decentration and dislocation due to the short steep scleral tunnel formed by the curved needle and the tip of the 20-gauge MVR blade. On the other hand, insufficient burial of the suture knot may cause suture-related complications. The second critical step in our technique is the cutting of the suture ends to make them as short as possible. We performed this step very carefully under a high magnification surgical microscope using 20- or 23-gauge microscissors. Thus, potential suture-related complications can be substantially prevented.
Our study had some limitations due to the development of a new scleral-fixation technique: its retrospective nature, the variable duration of follow-up, and the heterogeneous preoperative indications.
Scleral-fixation techniques need to cover, bury, or rotate suture knots to prevent overlying conjunctival erosion and suture-related complications. The “knot ball” scleral-fixation technique is a simple, less invasive, safe, and effective alternative method for transscleral fixation of SFIOLs. Our results were based on a short-term follow-up period. Prospective, randomized studies with longer follow-up periods are required to verify the safety and efficacy of primary and secondary SFIOL implantations using our new technique.
REFERENCES
1. Kongsap P. A knotless, one-haptic fixation of posterior chamber intraocular lenses: one-year results. Int J Ophthalmol. 2015;18;8(1): 104-6.
2. Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL; American Academy of Ophthalmology. Intraocular lens implantation in the absence of capsular support: a report by the American Academy of Ophthalmology. Ophthalmology. 2003;110(4):840-59.
3. McAllister AS, Hirst LW. Visual outcomes and complications of scleral-fixated posterior chamber intraocular lenses. J Cataract Refract Surg. 2011;37(7): 1263-9. Comment in: J Catarct Refract Surg. 2011;37(11):2089-90.
4. Apple D, Mamalis N, Loftfield K, Googe JM, Novak LC, Kavka-Van Norman D, et al. Complications of intraocular lenses: a historical and histopathological review. Surv Ophthalmol. 1984;29(1):l-54.
5. Sindal MD, Nakhwa CP, Sengupta S. Comparison of sutured versus sutureless scleral-fixated intraocular lenses. J Cataract Refract Surg. 2016;42(1):27-34.
6. Yalniz-Akkaya Z, Burcu A, Uney GO, Abay I, Eksioglu U, Acar MA, et al. Primary and secondary implantation of scleral-fixated posterior chamber intraocular lenses in adult patients. Middle East Afr J Ophthalmol. 2014;21(1):44-9. Comment in: Middle East Afr J Ophthalmol. 2014;21(4):366.
7. Lin CP, Tseng HY. Suture fixation technique for posterior chamber intraocular lenses. J Cataract Refract Surg. 2004;30(7):1401-4.
8. Hoffman RS, Fine IH, Packer M. Scleral fixation without conjunctival dissection. J Cataract Refract Surg. 2006; 32(11):1907-12.
9. Kir E, Kocaturk T, Dayanir V, Ozkan SB, Dündar SO, Aktunç TO. Prevention of suture exposure in transscleral intraocular lens fixation: an original technique. Can J Ophthalmol. 2008;43(6):707-11.
10. Baykara M. Suture burial technique in scleral fixation. J Cataract Refract Surg. 2004;30(5):957-9.
11. Bashshur Z, Ma’luf R, Najjar D, Noureddin B. Scleral fixation of posteriorchamber intraocular lenses using fascia lata to cover the knots. Ophthalmic Surg Lasers. 2002;33(6):445-9.
12. Bucci FA Jr, Holland EJ, Lindstrom RL. Corneal autografts for externalknots in transsclerally sutured posterior chamber lenses. Am J Ophthalmol. 1991;112(3):353-4.
13. Szurman P, Petermeier K, Aisenbrey S, Spitzer MS, Jaissle GB. Z-suture: a new knotless technique for transscleral suture fixation of intraocular implants. Br J Ophthalmol. 2010;94(2):167-9.
14. Agarwal A, Kumar DA, Jacob S, Baid C, Agarwal A, Srinivasan S. Fibrin glue assisted sutureless posterior chamber intraocular lens implantation in eyes with deficient posterior capsules. J Cataract Refract Surg. 2008;34(9):1433-8. Comment in: J Cataract Refract Surg. 2009;35(5):795; author reply 795-6.
15. Totan Y, Karadag R. Trocar-assisted sutureless intrascleral posterior chamber foldable intra-ocular lens fixation. Eye (Lond). 2012; 26(6):788-91.
16. Yamane S, Inoue M, Arakawa A, Kadonosono K. Sutureless 27-gauge needle-guided intrascleral intraocular lens implantation with lamellar scleral dissection. Ophthalmology. 2014;121(1):61-6. Comment in: Ophthalmology. 2014;121(8):e42-3. Ophthalmology. 2014;121(8):e42.
17. Chan TC, Lam JK, Jhanji V, Li EY. Comparison of outcomes of primary anterior chamber versus secondary scleral-fixated intraocular lens implantation in complicated cataract surgeries. Am J Ophthalmol. 2015;159(2):221-6.e2. Comment in: Am J Ophthalmol. 2015; 160(1):201-2. Am J Ophthalmol. 2015;160(1):201.
18. Luk AS, Young AL, Cheng LL. Long-term outcome of scleral-fixated intraocular lens implantation. Br J Ophthalmol. 2013;97(10):1308-11.
19. Hu XT, Zhang ZD, Zhou R, Pan QT. Cauterization technique for suture erosion in transscleral fixation of intraocular lenses. Int J Ophthalmol. 2013;18;6(6):892-4.
20. Por YM, Lavin MJ. Techniques of intraocular lens suspension in the absence of capsular/zonular support. Surv Ophthalmol. 2005; 50(5):429-62.
21. Friedberg MA, Berler DK. Scleral fixation of posterior chamber intraocular lenses combined with vitrectomy. Ophthalmic Surg. 1992; 23(1):17-21.
22. Almashad GY, Abdelrahman AM, Khattab HA, Samir A. Four-point scleral fixation of posterior chamber intraocular lenses without scleral flaps. Br J Ophthalmol. 2010;94(6):693-5.
23. Lin CP, Tseng HY. Suture fixation technique for posterior chamberintraocular lenses. J Cataract Refract Surg. 2004;30(7):1401-4.
24. Gabor SG, Pavlidis MM. Sutureless intrascleral posterior chamber intraocular lens fixation. J Cataract Refract Surg. 2007;33(11):1851-4. Comment in: Retina. 2014; 34(4):812-5.
25. Ohta T, Toshida H, Murakami A. Simplified and safe method of sutureless intrascleral posterior chamber intraocular lens fixation: Y-fixation technique. J Cataract Refract Surg. 2014;40(1):2-7. Comment in: J Cataract Refract Surg. 2014;40(5):851.
26. Lee VY, Yuen HK, Kwok AK. Comparison of outcomes of primary and secondary implantation of scleral fixated posterior chamber intraocular lens. Br J Ophthalmol. 2003;87(12):1459-62.
27. Lewis JS. Ab externo sulcus fixation. Ophthalmic Surg. 1991;22(11): 692-5.
Submitted for publication:
October 18, 2017.
Accepted for publication:
January 8, 2018.
Approved by the following research ethics committee: Gaziantep University Hospital (#187/2015)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose