

Luís Felipe da Costa Zulim1; Gisele Alborgetti Nai2, Rogério Giuffrida1; Carolina Silva Guimarães Pereira1; Hugo Benguella3; Aline Gutierrez Cruz3; Bruna Toledo Duran Foglia3; Aline da Silveira Batista4; Silvia Franco Andrade1
DOI: 10.5935/0004-2749.20180059
ABSTRACT
Objective: To compare the efficacy of 0.03% tacrolimus eye drops diluted in two different vehicles (linseed oil and olive oil) for the treatment of keratoconjunctivitis sicca (KCS) in dogs.
Methods: This study included 60 dogs. Of this group, 20 were healthy and allocated to the control group, and 40 were diagnosed with bilateral KCS and randomly allocated to either the TO (tacrolimus in olive oil) or the TL (tacrolimus in linseed oil) groups. Ophthalmic examinations, Schirmer Tear Test-1 (STT-1), Tear Film Break-up Time (TBUT) and Fluorescein Test (FT) were carried out monthly, along with cytological and histopathological examinations at the beginning and end of the study.
Results: The clinical signs, corneal ulcers, Schirmer Tear Test-1 values, and Tear Film Break-up Time values improved in both groups after one month of treatment. Cytological examination at the end of the study showed decreased lymphocytes, neutrophil, metaplastic, and squamous cell counts in both groups, while the histopathological analysis showed decreases in lymphocytes and neutrophils and an increase in goblet cell density (cells/mm2). The decreases in neutrophil count were more significant (p<0.05) in the TL group for both types of examination.
Conclusion: In sum, 0.03% tacrolimus eye drops diluted in olive oil and linseed oil were effective in the treatment of keratoconjunctivitis sicca. None of the evaluated parameters differed significantly between the two groups, except for neutrophil count which was significantly lower in the TL group. Thus, linseed oil may be considered as an alternative diluent for tacrolimus eye drops.
Keywords: Keratoconjunctivitis sicca; Tacrolimus; Olive oil; Linseed oil; Ophthalmic solutions; Animals; Dogs
RESUMO
Objetivo: Comparar a eficácia do tacrolimus 0,03% colírio, diluído em óleo de linhaça e óleo de oliva, no tratamento de ceratoconjuntivite seca em cães.
Métodos: Foram utilizados 60 cães; 20 cães saudáveis como grupo controle, e 40 cães com diagnóstico de ceratoconjuntivite seca bilateral, distribuídos aleatoriamente em dois grupos: Tacrolimus em óleo de oliva (TO) e Tacrolimus em óleo de semente de linhaça (TL). Os animais foram avaliados mensalmente com exames oftálmicos, Teste lacrimal de Schirmer-1 (TLS-1), Tempo de ruptura do filme lacrimal (TRFL) e Teste de Fluoresceína (TF), e mensalmente com citologia conjuntival e com exame histopatológico no início e final do estudo.
Resultados: Nos dois grupos de tratamento os sinais clínicos, Teste lacrimal de Schirmer-1, óleo de semente de linhaça e Tempo de ruptura do filme lacrimal apresentaram melhora após um mês de tratamento. E no final do estudo, na análise citológica, ambos apresentaram diminuição de linfócitos, neutrófilos, células metaplásicas e células escamosas, e na análise histopatológica houve diminuição de linfócitos, neutrófilos e o aumento de células caliciformes. No grupo óleo de semente de linhaça, a diminuição de neutrófilos foi mais significativa (p<0,05) em ambas análises.
Conclusão: Em suma, tacrolimus 0,03% colírio diluído em óleo de oliva e óleo de linhaça foram eficientes no tratamento de ceratoconjuntivite seca. Nenhum dos parâmetros avaliados diferiu significativamente entre os dois grupos, exceto a contagem de neutrófilos, que foi significativamente menor no grupo TL. Assim, o óleo de linhaça pode ser considerado como um diluente alternativo para o colírio tacrolimus.
Descritores: Ceratoconjuntivite seca; Tacrolimus; Azeite de oliva; Óleo de semente do linho; Soluções oftálmicas; Animais; Cães
INTRODUCTION
Keratoconjunctivitis sicca (KCS) is a chronic, inflammatory ophthalmic disorder that commonly occurs in humans and dogs. It is characterized by a quantitative decrease in the aqueous layer of the tear film and/or a qualitative deficiency in the lipid or mucin layer that leads to a progressive inflammatory process primarily affecting the cornea, conjunctiva, and lacrimal glands. Its origin is usually immunomediated(1-3).
Canine models are excellent for developing an understanding of this disease as the symptomatology in dogs is quite similar to that observed in humans. The clinical signs of canine KCS include conjunctival hyperemia, chemosis, blepharospasm, photophobia, mucoid and mucopurulent ocular secretion, corneal ulcer, vascularization and pigmentation of the cornea, and loss of vision(3,4). Certain canine breeds such as the English Bulldog, Lhasa Apso, and Cocker Spaniel are predisposed to KCS, particularly in females.
Topical therapy for KCS predominantly consists of immunosuppressants, including cyclosporine, tacrolimus, and pimecrolimus, used in association with lubricants. Anti-inflammatory agents, antibiotics and mucolytics may also be used if considered necessary(4-12). Previous studies have reported benefits associated with the adjunctive use of oral or topical omegas 3 (ω-3) and 6 (ω-6) for the treatment of KCS as they restore the lipid layer, decrease inflammation and apoptosis, and increase tear secretion(13-21).
Tacrolimus (FK506) is a macrolide antibiotic that is isolated from Streptomyces tsukubaensis. Its effects are similar to cyclosporine, and include a combination of local immunosuppression, goblet cell proliferation, suppression of lacrimal cell apoptosis, and anti-inflammatory action(9-11). It is common for tacrolimus or cyclosporine eye drops to be diluted in olive oil or almond oil(9,20-22), which are rich in essential fatty acids (EFAs), such as ω-3 and ω-6, that act as natural anti-inflammatory agents. Alpha-linolenic acid (ALA), an ω-3 fatty acid, and linoleic acid (LA), an ω-6 fatty acid, compete for metabolism by the enzyme Δ6-desaturase, and convert into omegas with anti-inflammatory properties. ALA gives rise to the ω-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), while LA gives rise to the ω-6 fatty acids dihomo-gamma-linolenic acid (DGLA), prostaglandin E1 (PGE1), and thromboxane (TXA1)(13-16).
Olive oil, derived from Olea europeae, contains large quantities of monounsaturated fatty acids (MUFAs), 70-80% oleic acids, 10-15% saturated fatty acids (palmitic acid), and a small quantity of polyunsaturated fatty acids (PUFAs) including approximately 5 to 10% of omegas 3, 6, and 9. It has anti-inflammatory, antinociceptive, immunomodulatory, and antimicrobial properties(23,24). A study comparing the treatment of experimentally induced KCS in rabbits using olive oil alone or in combination with cyclosporine reported that the former exhibited considerable ability to control the symptoms of the disease(21).
Linseed oil is considered one of the most abundant sources of the EFAs, ω-3 and ω-6, with the ratio of ω-6 to ω-3 being close to ideal at 1:3(25). Oral administration of this oil has been recommended as an adjuvant therapy for Sjögren KCS syndrome in human beings(13,14). Moreover, use of linseed oil, both alone (orally, topically or a combination of the two) and in association with tacrolimus and cyclosporine, has also been reported to have improved the symptoms of experimentally induced KCS in rabbits(15,19-21).
The objective of this study was to compare the efficacy of tacrolimus 0.03% eye drops diluted in olive oil (commonly used for this purpose) to those diluted in linseed oil, which has not been tested previously as a tacrolimus diluent for the treatment of KCS in dogs.
METHODS
Animals
The study was approved by the Ethics Committee on Animal Use (CEUA) of UNOESTE (protocol no. 1794), and it was undertaken in accordance with the rules of ARVO (Association for Research in Vision and Ophthalmology-Statement for the Use of Animals in Ophthalmic and Visual Research). The study included forty dogs [25 females (62.5%) and 15 males (37.5%)] with bilateral KCS that belonged to clients attending the university veterinary hospital. The mean age of the dogs was 6.7 ± 3.9 years, and the mean weight was 10.3 ± 7.7 kg. The inclusion criteria were presence of typical ophthalmic clinical signs (ocular discharge, conjunctivitis, corneal opacity, and pigmentation), Schirmer Tear Test-1 (STT-1) values that were <10 mm/min, and/or Tear Film Break-up Time (TBUT) values that were <10 sec. The negative control group consisted of twenty healthy mixed breed dogs [9 males (45%) and 11 females (55%)] selected from the university kennel. Their mean age was 3.5 ± 2.4 years and the mean weight was 10.3 ± 7.7 kg. Ophthalmic examination of the dogs included slit-lamp bio-microscopy (SL-15 Kowa, Japan), STT-1, TBUT, and cytological and conjunctival histopathological examination.
Groups
After diagnosis of KCS, the dogs were randomly divided into two groups, as follows: a) TO group (n=20): tacrolimus 0.03% eye drops diluted in olive oil (Ophthalmos Laboratory, São Paulo, Brazil), and b) TL group (n=20): tacrolimus 0.03% eye drops diluted in linseed oil (Ophthalmos Laboratory, Sao Paulo, Brazil). Double-blinding was maintained throughout the course of this study. Treatment in both groups consisted of application of the respective tacrolimus combinations twice daily in both eyes. Both groups also received 1 drop of propylene glycol-based lubricant (Systane®, Alcon, São Paulo, Brazil) in both eyes twice daily for six months.
Antibiotic eye drops (1 drop 4 times per day for 15 days) were used when cultures of ocular samples secretions from the dogs demonstrated antimicrobial sensitivity and the presence of corneal ulcers or clinical signs such as conjunctivitis and mucopurulent ocular secretions were observed. Anti-inflammatory eye drops containing diclofenac sodium (Still®, Allergan, São Paulo, Brazil) were only used in case of eye discomfort and ocular hyperemia without corneal ulcers [TO group: 70% (28/40), TL group: 57.5% (23/40)]. Melting ulcers (keratomalacia) were observed in 2.5% (1/40) of the TO group and 2.5% (1/40) of the TL group and were treated using anti-collagenase treatment with equine serum (1 drop 6 times per day for 15 days). The TO and TL combinations were formulated free of preservatives, stored at room temperature, and protected from the sun.
Ophthalmic examinations
Ophthalmic and cytological examinations were performed monthly, with the first day representing baseline (M0) and all subsequent treatments being considered as follow-up time-points (M1 to M6). Histopathological examinations were performed at the time of diagnosis (M0) and study completion (M6). Specific scores for all ophthalmologic clinical signs were assigned by the same examiner (LFCZ), as follows: conjunctivitis (0-none; 1-mild conjunctival hyperemia; 2-moderate to severe conjunctival hyperemia; 3-moderate to severe conjunctival hyperemia and chemosis); ocular discharge (0-none; 1-minor serous discharge; 2-moderate mucoid discharge; 3-marked mucopurulent discharge); corneal opacity (0-none/absent;1-nebula/minor, diffuse and hazy opacity with indistinct borders, less than 25% extension of the cornea; 2-macula/moderately dense opacity with circumscribed border, between 25% and 50% extension of the cornea; 3-leukoma/dense and white opacity, more than 50% extension of the cornea); and corneal pigmentation (0-none; 1-less than 25%; 2-between 25% to 50%; and 3-more than 50%). Measurements in the control group, carried out at M0, were considered to be the “normal” parameters for the ophthalmic examinations conducted in the case group.
The Schirmer Tear Test-1 (STT-1) (Teste de Schirmer® - Ophthalmos Laboratory, São Paulo, Brazil), used to quantify the tears, was always performed at the same time (between 2 and 3 at pm) and without any anesthetic drops. A score of <10 mm/min was considered diagnostic for KCS (26). The TBUT was used to qualitatively evaluate the tear film. After placing a drop of 1% fluorescein eye drops (Fluoresceína®; Allergan, Sao Paulo, Brazil) in the lower fornix, a slit-lamp with a bright light setting and a cobalt blue filter was used to measure the time between the last blink and the first appearance of a dark spot on the cornea (formation of a dry area). TBUT values <10 seconds were considered diagnostic for KCS (27).
The fluorescein test (FT) was performed using 1% fluorescein eye drops (Fluoresceína®; Allergan, São Paulo, Brazil), and the presence or absence of corneal ulcers were evaluated with the help of slit-lamp bio-microscopy (SL-15, Kowa, Japan) using a cobalt blue filter(26). Scores were assigned based on the severity, extension, or depth of the ulcer (0-negative, 1- small superficial ulcer, 2- medium superficial ulcer, 3- extensive surface ulcer, 4-small stromal ulcer, 5-medium stromal ulcer, 6-extensive stromal ulcer, 7-descemetocele, and 8-keratomalacia).
Cytological and histological examinations
The cytological examinations were performed by the same person throughout the course of the study. The eye was cleaned with saline and anesthetic eye drops (0.5% proxymetacaine, Anestalcon®; Allergan, São Paulo, Brazil) were then applied. Samples from the lower palpebral conjunctival cells were harvested using a sterile swab moistened with saline and a microscope glass slide. The samples were then fixed in methanol and stained using the MGG technique (May-Grunwald-Giemsa). Lymphocytes, neutrophils, metaplastic cells, and squamous cells were counted in 10 fields under an optical microscope at 40x objective.
The conjunctival biopsy was performed after application of anesthetic eye drops (0.5% proxymetacaine, Anestalcon®, Allergan, Sao Paulo, Brazil). Specimens measuring 1-3 mm were harvested from the palpebral portion of the inferior medial conjunctival fornix using forceps and conjunctiva scissors (HR, São Paulo, Brazil). The histological section was placed on a fragment of paper (1x1 cm in size), fixe d in formalin, and then embedded in paraffin (Dinâmica Reagentes Analíticos, São Paulo, Brazil). A rotary microtome was used to create 5-μm sections of the specimen, and these were then stained using hematoxylin and eosin (HE) (Dolles, São Paulo, Brazil) and Periodic acid-Schiff stain (PAS) (Merck, USA). The HE stain was used to count the lymphocytes and neutrophils, while the PAS stain was used to measure the goblet cell density (cells/mm2). A Nikon Eclipse E200 (Tokyo, Japan) optical microscope at 40X objective was used for all cytological and histological cell counting, and the Leica ICC50HD (Wetzlar, Germany) was used for all light microscopy examinations.
Statistical analysis
The two-way analysis of variance (ANOVA) for paired samples with post-hoc Tukey’s test were used to analyze the STT-1 and TBUT variables, including goblet cell density and the number of squamous cells, metaplastic cells, lymphocytes, and neutrophils. With regard to the FT variables, the Friedman’s non-parametric test was used to compare various time points, while the Kruskal-Wallis test with post-hoc Dunn’s test was used to compare the variables between groups. Statistical significance was set at p<0.05, and all analyses were performed using the statistical package R, version 3.2.2. (The R Foundation for Statistical Computing, 2015).
RESULTS
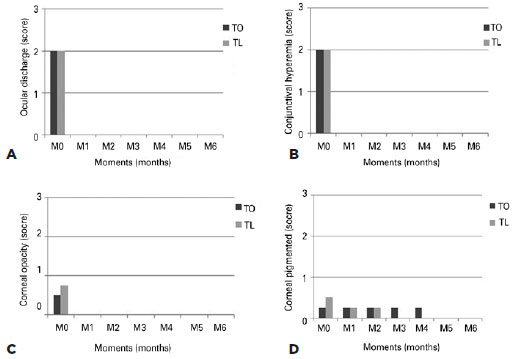
The normal parameters, defined by the negative control group, are shown in table 1. Both treatment groups (TO and TL) exhibited improvements in the clinical signs (Figure 1), and no statistically significant differences (p>0.05) between the groups were observed. However, the findings showed significant differences (p<0.05) between M0 and the other time points. Complete remission of ocular discharge, conjunctivitis, and corneal opacity were observed in both treatment groups at M1, while the median for corneal pigmentation exhibited complete remission by M5.
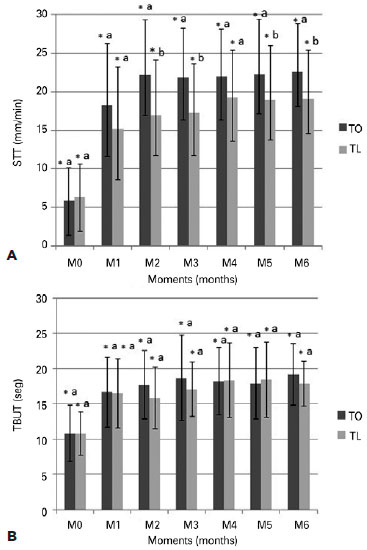
The STT-1 (Figure 2) showed that the TO and TL groups exhibited significant increases at M1 (TO: 18.2 ± 6.6, p=0.9; TL: 15.1 ± 5.5, p=0.5). Moreover, statistically significant differences were observed at M2 (p=0.002) and M3 (p=0.007), with the TO group exhibiting higher values compared to the TL group. Both groups demonstrated statistically significant differences (p<0.05) in STT-1 values between M0 and all other time points although overall the values were similar to those observed in the negative control group (Table 1). The TBUT (Figure 2) showed that the two groups exhibited significant increases at M1 (TO: 16.6 ± 4.3, p=0.48; TL: 15.8 ± 3.4, p=0.6) that continued until M6 (TO: 19.2 ± 3.6, p=0.27; TL: 17.9 ± 2.6, p=0.34), and the values were similar to those observed in the negative control group (Table 1). Although no differences (p>0.05) in TBUT values were observed between the groups, a significant difference was seen when comparing M0 with the other time points.
The FT showed that the ulcers observed in the two groups at M0 differed in terms of severity and extent [TO: 30% (12/40), TL: 42.5% (17/40)]. Excellent healing of all ulcers was observed in both groups at M1 [TO: 91.7% (11/12), TL: 94.1% (16/17)], except for keratomalacia [TO: 8.3% (1/12), TL: 5.9% (1/17)] which only completely resolved in both groups at M2. No statistically significant differences (p>0.05) were observed.
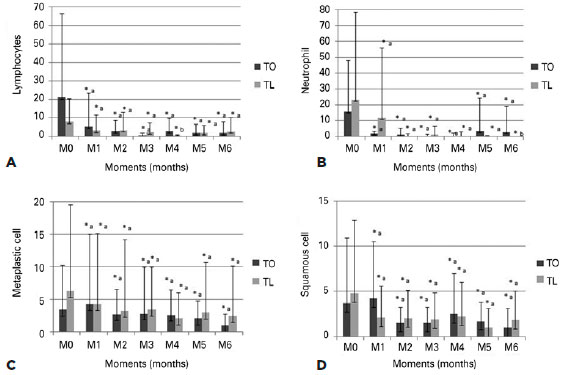
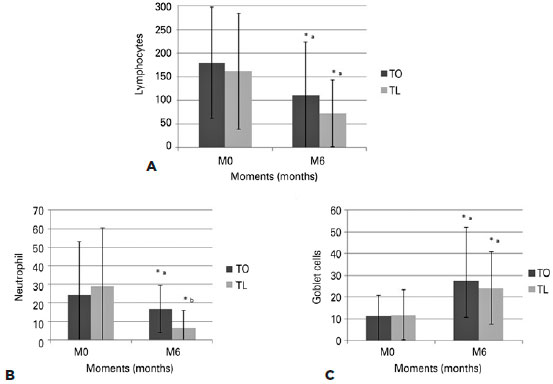
The cytological examination (Figure 3) showed decreases in all cell counts in both groups. No statistically significant differences were observed between the groups, except with regard to the neutrophils, which were significantly lower in the TO group compared to the TL group at time point M1 (p=0.036) and in the TL group compared to the TO group at time-point M6 (p=0.029). The histopathological examination (Figure 4) showed that both groups exhibited decreases in the inflammatory cell counts, with the neutrophil count (p=0.0016) in the TL group being significantly lower than that in the TO group at M6. An overall increase in the number of goblet cells (p=0.012) was observed in both groups, with no statistically significant differences between the two (p=0.6). However, significant differences were seen between time points M0 and M6 (TO: p=0.012, TL: p=0.005).
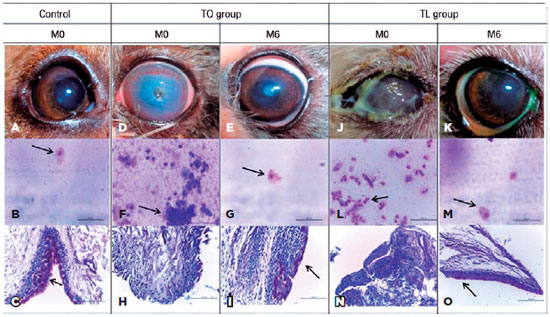
Therefore, the results showed that both groups exhibited improvements overall, with the observed values being close to the ‘normal’ parameters defined by the negative control group (Table 1). The aspects of some eyes and cytological and histopathological examinations from the control group, TO group and TL group are shown in figure 5.
DISCUSSION
In this study, both treatment groups demonstrated improvements in the clinical signs of KCS, increased STT-1 and TBUT values, and resolution of corneal ulcers. These improvements could be a result of the proven effects of tacrolimus (local immunosuppression, goblet cell proliferation, suppression of lacrimal cell apoptosis, and anti-inflammatory action)(9-11), as well as the possible additional effects of the oils added to the formula. The ω-3 and ω-6 fatty acids present in these oils inhibit the formation of pro-inflammatory eicosanoids and increase the formation of anti-inflammatory mediators such as EPA, DHA, prostaglandin E1 (PGE1), and TXA1(13-18).
Studies examining the treatment of experimentally induced KCS in rabbits using common topical immunosuppressants (cyclosporine and tacrolimus) diluted in vegetable oils (almond, olive oil, and linseed oil), as well as those focusing on the isolated use of these oils, reported that both the immunosuppressants and the oils exhibited efficacy in controlling the symptoms of KCS. This was particularly true with regard to the ω-3 and ω-6 fatty acids, which induced formation of anti-inflammatory mediators such as PGE1 A1 and thromboxane 1 (TXA 1)(19-21). Most of these studies reported better results with linseed oil compared to any of the other oils, including almond(20) and olive(21), possibly due to its higher ω-3 and ω-6 content(19-25).
Another study examining the treatment of experimentally induced KCS in rabbits using linseed oil through various routes (oral, topical, and oral and topical in combination) reported that it was effective in controlling the symptoms of the disease and increasing the goblet cell counts(19). Although these experimental studies used rabbits in their KCS induction protocols with topical atropine combined or not with third eyelid gland removal, was different from our study, in which KCS was studied in dogs with a natural immune-mediated disease, all of these studies serve as a basis for better understanding this disease.
Beagle dogs treated with tacrolimus diluted in olive oil exhibited an improvement in clinical signs (secretion, pigmentation, and hyperemia). This did not differ significantly from the effects observed with another immunosuppressant, cyclosporine. A previous study also reported a significant increase in the STT-1 values at the first time-point, and this was similar to the findings of the current study(9).
The STT-1 and TBUT values significantly increased over the study period, although no statistically significant differences between the TO and TL groups were observed. This was in agreement with another study that used 0.02% cyclosporine diluted in olive oil and linseed oil to treat experimentally induced KCS in rabbits(21). Conversely, another study using 0.03% tacrolimus diluted in almond oil and linseed oil reported significant improvement only in the group with topical application of linseed oil(20). Significantly higher STT-1 values were also observed in another study using oral and topical linseed oil for the treatment of KCS in rabbits(19).
In the current study, excellent resolution of corneal ulcers was observed in both groups (TO and TL), and there were no statistically significant differences between the two. This was in agreement with several other studies(20,21), including one that reported resolution of keratomalacia (“melting” corneal ulcers) upon oral and topical administration of linseed oil for the treatment of KCS in rabbits(19). The present study also included several other substances such as lubricants, antibiotics and equine serum for the treatment of ulcers, and this differed from the studies focusing on experimentally induced KCS in rabbits, which primarily used immunosuppressive agents and/or vegetable oils only.
The neutrophil count at the end of treatment was seen to differ significantly between the two groups in the current study, with numbers being considerably lower in the TL group compared to the TO group. A previous study also reported a decrease in the number of inflammatory cells following treatment of KCS in dogs using 2% topical cyclosporine(28). The literature suggests that the pathogenesis of KCS can be attributed to immune-mediated inflammation and destruction of the lacrimal glands, characterized by inflammatory infiltrates such as lymphocytes and, to a lesser degree, neutrophils(1,2,29).
The present study showed a significant increase in the goblet cell density between the start and end of treatment, with no significant differences observed between the groups. This was in agreement with previous studies that also reported an increase in goblet cells following treatment using immunosuppressants(20,21) or omega fatty acids(13,19).
In conclusion, the results of this study showed that 0.03% tacrolimus eye drops diluted in olive oil (TO) and linseed oil (TL) was effective for the treatment of KCS in dogs. There were no statistically significant differences between the two treatment groups, except with regard to the neutrophil count which was greater in the TL group. This suggested that linseed oil could be used as an alternative diluent for tacrolimus eye drops.
REFERENCES
1. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immunemediated ocular surface disorder. Arch Ophthalmol. 2012; 130(1):90-100. Comment in: Ophthalmologe. 2014;111(5):411.
2. McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57(4):293-316.
3. Williams DL. Immunopathogenesis of keratoconjunctivitis sicca in the dog. Vet Clin North Am Small Anim Pract. 2008;38(2):251-68.
4. Carter R, Colitz CM. The causes, diagnosis, and treatment of canine keratoconjunctivitis sicca. Vet Med. 2002;97(9):683-94.
5. Grahn BH, Storey ES. Lacrimomimetics and lacrimostimulants. Vet Clin North Am Small Anim Pract. 2004;34(3):739-53.
6. Izci C, Celik I, Alkan F, Ogurtan Z, Ogurtan Z, Ceylan C, et al. Histologic characteristics and local cellular immunity of the gland of the third eyelid after topical ophthalmic administration of 2% cyclosporine for treatment of dogs with keratoconjunctivitis sicca. Am J Vet Res. 2002;63(5):688-94.
7. Yavuz B, Bozdaq Pehlivan S, Ünlü N. An overview on dry eye treatment: approaches for cyclosporine A delivery. Scientific World J. 2012;2012:194848.
8. Izci C, Celik I, Alkan F, Erol M, Sur E. Clinical and light microscopic studies of the conjunctival tissues of dogs with bilateral keratoconjunctivitis sicca before and after treatment with topical 2% cyclosporine. Biotech Histochem. 2015;90(3):223-30.
9. Hendrix VD, Adkins EA, Ward, DA, Stuffle J, Skorobohach B. An investigation comparing the efficacy of topical ocular application of tacrolimus and cyclosporine in dogs. Vet Med Int. 2011;2011: 487592.
10. Berdoulay YA, English RV, Naldelstein B. Effect of topical 0.02% tacrolimus aqueous suspension on tear production in dog with keratoconjunctivitis sicca. Vet Ophthalmol. 2005;8(4):225-32.
11. Moskovici BK, Holzchuh R, Sakassegawa-Naves FE, Hoshino-Ruiz DR, Albers MB, Santo RM, et al. Treatment of Sjögren’s syndrome dry eye using 0.03% tacrolimus eyedrop: Prospective double-blind randomized study. Cont Lens Anterior Eye. 2015;38(5):373-8.
12. Ofri R, Lambrou GN, Allgoewer I, Graenitz U, Pena TM, Spiess BM, et al. Clinical evaluation of pimecrolimus eye drops for treatment of canine keratoconjunctivitis sicca: a comparison with cyclosporine A. Vet J. 2009;179(1):70-7.
13. Barabino S, Rolando M, Camicione P, Ravera G, Zanardi S, Giuffrida S, et al. Systemic linoleic and γ-linolenic acid therapy in dry eye syndrome with an inflammatory component. Cornea. 2003; 22(2):97-101.
14. Aragona P, Bucolo C, Spinella R, Giuffrida S, Ferreri G. Systemic omega-6 essential fatty acid treatment and PGE1 tear content in Sjögren’s syndrome patients. Invest Ophthalmol Vis Sci. 2005; 46(12):4474-9.
15. Rashid S, Jin Y, Ecoiffer T, Barabino S, Schaumberg DA, Dana MR. Topical omega-3 and omega-6 fatty acids for treatment of dry eye. Arch Ophthalmol. 2008;126(2):210-25.
16. Roncone M, Bartlett H, Eperjesi F. Essential fatty acids for dry eye: A review. Cont Lens Anterior. 2010;33(2):49-54.
17. Wojtowicz, JC, Butovich I, Uchiyama E, Aronowicz J, Agee S, Mc- Culley JP, et al. Pilot, prospective, randomized, double-masked, placebo-controlled clinical trial of an omega-3 supplement for dry eye. Cornea. 2011;30(3):308-14.
18. Barghara R, Kuman P, Kuman M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6(6):811-3.
19. Neves ML, Yamasaki L, Sanches O de C, do Amaral MS, Stevanin H, Giuffrida R, et al. Use of linseed oil to treat experimentally induced keratoconjunctivitis sicca in rabbits. J Ophthalmic Inflamm Infect. 2013;3(1):4.
20. Sgrignoli MR, Yamasaki L, Sanches OC, Giuffrida, R, Ricci, CL, Santos GC, et al. Comparison of topical 0.03% tacrolimus in almond and linseed oil to treat experimentally induced keratoconjunctivitis sicca in rabbits. Int J Ophthalmic Pathol. 2013;2(3). doi:10.4172/2324- 8599.1000114
21. Parrilha LR, Nai GA, Giuffrida R, Barbero RC, Padovani LD, Pereira RH, et al. Comparison of 1% cyclosporine eye drops in olive oil and in linseed oil to treat experimentally-induced keratoconjunctivitis sicca in rabbits. Arq Bras Oftalmol. 2015;78(5):295-9.
22. Benitez del Castillo JM, Del Aguila C, Duran S, Hernandez J, Garcia Sanchez J. Influence of topically applied cyclosporine A in olive oil on corneal epithelium permeability. Cornea. 1994;13(2):136-40.
23. Waterman E, Lockwood B. B. Active components and clinical applications of olive oil. Alter Med Rev. 2007;12(4):331-42.
24. Eidi A, Moghadam-Kia S, Moghadam JZ, Eidi M, Rezazadeh S. Antinociceptive and anti-inflammatory effects of olive oil (Olea europeae L.) in mice. Pharm Biol. 2012;50(3):332-7.
25. Hassan-Zadeh A, Sahari MA, Barzegar M, Optimization of the -3 extraction as a functional food from flaxseed. Int J Food Sci Nutr. 2008;59(6):526-34.
26. Maggs DJ. Basic diagnostic techniques. In: Maggs DJ, Miller PE, Ofri R, editors. Slatter’s Fundamentals of Veterinary Ophthalmology. St. Louis: Saunders Elsevier; 2008. p. 81-106.
27. Saito A, Kotani T. Estimation of lacrimal level and testing methods on normal beagles. Vet Ophthalmol. 2001;4(1):7-11.
28. Izci C, Celik I, Alkan F, Ogurtan Z, Ceylan C, Sur E, et al. Histologic characteristics and local cellular immunity of the gland of the third eyelid after topical ophthalmic administration of 2% cyclosporine for treatment of dogs with keratoconjunctivitis sicca. Am J Vet Res. 2002;63(5):688-94.
29. Conrady CD, Joos ZP, Patel BC. Review: The lacrimal gland and its role in dry eye. J Ophthalmol. 2016;2016:7542929.
Submitted for publication:
April 26, 2017.
Accepted for publication:
February 8, 2018.
Approved by the following research ethics committee: Ethics Committee on the Use of Animals (CEUA) of UNOESTE (# 1794)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: The authors have no potential conflicts of interest to disclose