

Burcu Dirim1; Selam Yekta Sendul1; Semra Tiryaki Demir1; Hakan Kacar1; Ali Olgun1; Erdem Ergen2; Saniye Uke Uzun1; Dilek Guven1
DOI: 10.5935/0004-2749.20180056
ABSTRACT
Purpose: To evaluate peripapillary choroidal thickness changes in contralateral eyes of patients who had undergone evisceration of their diseased eyes.
Methods: In this retrospective study, peripapillary choroidal thickness parameters in 34 eyes of 34 patients who had undergone diseased-eye evisceration between March 2014 and May 2016 were evaluated using spectral domain optical coherence tomography. The scans were manually delineated to identify the principal surfaces of Bruch’s membrane, the Bruch’s membrane opening, and the anterior sclera. Peripapillary choroidal thickness was measured between the Bruch’s membrane and the anterior sclera at increasing distance away from the Bruch’s membrane opening. The mean peripapillary choroidal thickness values in the contralateral eyes of the patients and those of the control group were compared.
Results: The mean peripapillary choroidal thickness was higher in the contralateral eyes of the patients compared with that of normal eyes at all distances from the Bruch’s membrane opening.
Conclusion: Increased peripapillary choroidal thickness was noted in the contralateral eyes of the patients, potentially resulting in a thicker choroid. Although further investigation is required to determine the cause, these findings indicate the presence of a compensatory factor in the contralateral eyes.
Keywords: Eye evisceration; Optical coherence tomography; Choroid/pathology
RESUMO
Objetivo: Avaliar as alterações da espessura coroide peripapilar em olhos contralaterais de pacientes submetidos à evisceração do olho doente.
Métodos: Neste estudo retrospectivo, parâmetros da espessura coróide peripapilar de 34 olhos de 34 pacientes submetidos à evisceração, entre março de 2014 e maio de 2016, foram avaliados com tomografia de coerência óptica de domínio espectral. As varreduras foram manualmente delineadas para identificar as principais superfícies da membrana de Bruch, a abertura da membrana de Bruch e a esclera anterior. A espessura coroide peripapilar foi medida entre a membrana de Bruch e a esclera anterior a uma distância crescente da abertura da membrana de Bruch. Compararam-se os valores médios da espessura coroide peripapilar dos olhos contralaterais dos pacientes e do grupo controle.
Resultados: A espessura coroide peripapilar média foi mais espessa nos olhos contralaterais dos pacientes, quando comparada com os olhos normais, em todas as distâncias da abertura da membrana de Bruch.
Conclusão: O aumento da espessura coroide peripapilar foi notado nos olhos contralaterais dos pacientes. O espessamento da coroide pode ser resultante do distúrbio. Embora seja necessária uma investigação mais aprofundada para determinar a causalidade, esses achados podem apontar para um fator compensatório dos olhos contralaterais.
Descritores: Evisceração do olho; Tomografia de coerência óptica; Coroide/patologia
INTRODUCTION
Evisceration is a surgical procedure in which the contents of the eye are removed while preserving the sclera, Tenon’s capsule, conjunctiva, and optic nerve. The choroid is a multifunctional vascular tissue of the eye with the primary function of supplying oxygen to the outer retina and the optic nerve head. In addition, the choroid tissue has one of the highest rates of blood flow in the human body, and the vascular supply in this tissue is derived primarily from long and short posterior ciliary arteries of the ophthalmic artery, with some contribution from the anterior ciliary arteries(1). Evisceration affects the ophthalmic artery blood flow in the diseased eye, which may cause a change in choroid thickness in both eyes.
The choroid plays an important role in the physiology of healthy eyes and in the pathogenesis of a variety of ocular diseases. Hemodynamic studies using various techniques have demonstrated alterations in choroidal blood flow in diseased eyes(2-4). Because choroidal thickness changes with specific pathologies, measurements of choroidal thickness are becoming more common and are accepted procedures in clinics and in research(5-8).
Advancement in spectral-domain optical coherence tomography (SD-OCT) imaging technology has allowed visualization and reproducible quantification of the optic nerve head and choroid structures(9-12).
The aim of this study was to compare peripapillary choroidal thickness (PCT) in the contralateral eyes of patients who had undergone evisceration surgery, with that in healthy eyes of a control group. We hypothesized that evisceration of a diseased eye would affect choroidal thickness of the contralateral eye and evaluated PCT values in these eyes using SD-OCT to identify changes compared to the control group.
METHODS
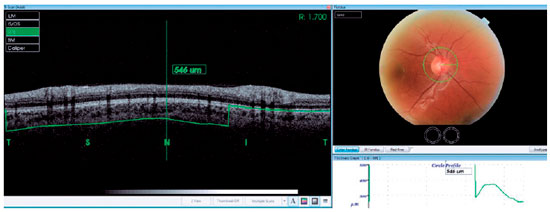
Thirty-four patients who had a history of unilateral evisceration at Sisli Hamidiye Etfal Training and Research Hospital from March 2014 to May 2016 were reviewed retrospectively. Comparative participants who had no ocular diseases were included as controls. High-resolution SD-OCT (3DOCT-2000; Topcon Inc., Tokyo, Japan) was used to evaluate PCT. Thirty-four normal eyes of patients and 34 eyes of healthy controls were subject to a complete ophthalmological examination including medical history, Snellen’s best-corrected visual acuity, slit lamp biomicroscopy, applanation tonometry, and dilated fundoscopy. We selected 3D-OCT circle scan images used to capture retinal nerve fiber layer (RNFL) images in a 3-4-mm zone around the optic disc. The PCT was measured manually and perpendicularly from the outer edge of the hyperreflective retinal pigment epithelium or the Bruch’s membrane opening (BMO) to the choroid-sclera boundary at the nasal, temporal, superior, and inferior quadrants (Figure 1). All SD-OCT measurements were obtained by the same clinician. Exclusion criteria included a diagnosis of open-angle glaucoma, intraocular pressure (IOP) greater than 21 mmHg, presence of other ocular diseases, or previous intraocular surgery. The study was conducted in accordance with the tenets of the Declaration of Helsinki by obtaining written consent from all patients, with the approval of the local ethical review board.
Statistical analysis
For descriptive statistics of the data, mean, standard deviation, or median of the lowest and highest values was used. The distribution of the variables was measured using the Kolmogorov-Smirnov test. For analysis of quantitative data, the Mann-Whitney U test and the independent-samples t-test were used. The software SPSS 22.0 was used for the analyses.
RESULTS
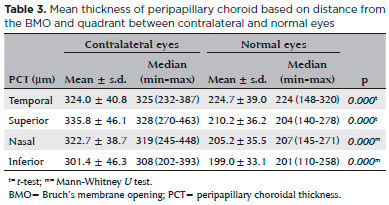
Thirty-four eyes were operated at our Hospital from March 2014 to May 2016. The demographic data and clinical characteristics of the eyes are summarized in table 1. Indications for surgery are shown in table 2. The most common reason of surgery was traumatic phthisis. The age and gender of the subjects were similar (p>0.05). As shown in table 1, the age of the participants in the patient group ranged between five and 84, with a mean age of 40.5 years. The age of participants in the healthy group ranged between 36 and 57, with a mean age of 43. Among the 34 patients, 26 (76.4%) were male and eight (23.5%) were female. The PCT values in the contralateral eyes in the temporal, superior, nasal, and inferior quadrants were 324.0 ± 40.8 µm, 335.8 ± 46.1 µm, 322.7 ± 38.7 µm, and 301.4 ± 46.3 µm, respectively. Furthermore, the PCT values in normal eyes in the four quadrants were 224.7 ± 39 µm, 210.2 ± 36.2 µm, 205.2 ± 35.5 µm, and 199.0 ± 33.1 µm, respectively.
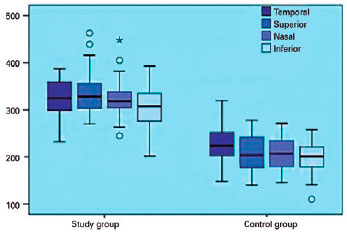
Thus, the PCT values in the contralateral eyes were significantly higher than those in the normal eyes at all quadrants, as shown in figure 2 and table 3 (p<0.05).
DISCUSSION
In this study, we demonstrated that contralateral eyes of patients who had undergone evisceration had greater PCT than those of the control group, indicating increased choroidal circulation in the patients. The cause of this mean total increase is unknown. We found that the PCT values in all quadrants and peripapillary sectors provided by classic topographic OCT analysis were increased in the patients compared with those in the healthy controls.
Evisceration involves the removal of the contents of the globe of the eye, leaving the sclera, extraocular muscles, and optic nerve intact. One of the advantages of evisceration is minimal disruption of the orbital anatomy. Thus, the chances of injuring the vascular system and nerves are reduced due to minimal dissection within the orbit.
In the evisceration operation, the ophthalmic artery and vortex veins are cauterized; therefore, the thickening of the peripapillary choroid we observed might be related to the expansion of peripapillary choroidal blood capillaries. Choroidal perfusion is provided by both long and short posterior ciliary arteries, and perforating anterior ciliary arteries of ophthalmic origin. Further, a combination of anterior and posterior communicating arteries constitutes the circle of Willis which permits collateral flow between the two ophthalmic arteries.
Vascular ultrastructural alterations following surgery may affect choroidal thickness. We suggest that vascular flow in the ophthalmic artery in the eviscerated eye contributes to that in the contralateral ophthalmic artery through the Willis circle following surgery, and causes increased choroid thickness in the contralateral eye.
The choroid is a dynamic structure and its thickness depends on various factors, including age, race(13), glaucoma(14-16), and axial length(17,18). In the present study, however, we excluded patients with high IOP or high myopia. Dayanir et al. have found that the values of ophthalmic artery peak systolic velocity and end diastolic velocity were lower in patients with unilateral pseudoexfoliative syndrome, resulting in decreased PCT compared with those in the control group(19). The choroid was also found to be thicker in the contralateral eyes in our study, suggesting blood supply to the ophthalmic artery of the contralateral eyes. Further, Kantarci et al. found greater PCT in adult anisometropic amblyopic eyes compared with contralateral eyes, as in our study(20). We suggest that the anatomic and functional changes in the contralateral eyes of anophthalmic and amblyopic patients may be similar to those we found.
The strength of our study is that it is the first study to analyze choroidal thickness comparing eyes of anophthalmic patients with those of a control group. However, our study could not determine whether the choroidal thickness change found occurred before the evisceration. This study also had other limitations. First, the choroid can be influenced by circadian changes(21), and we did not perform OCT examinations at the exact time of day in all participants. We also did not establish the reliability of automated choroidal thickness measurements using the recently developed EDI or SS-OCT.
Therefore, further investigations involving a larger number of patients are needed to confirm the difference in choroidal thickness between the contralateral eyes of anophthalmic patients and healthy eyes.
In conclusion, the choroidal thickness in the contralateral eyes of eviscerated patients was higher than that in eyes of healthy subjects. This suggests that increased choroidal circulation may be a morphologic feature associated with vascular compensatory expansion in the ophthalmic artery and peripapillary choroidal blood capillaries following evisceration.
REFERENCES
1. Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Progr Retin Eye Res. 2012;31(5):377-406.
2. Deokule S, Vizzeri G, Boehm AG, Bowd C, Medeiros FA, Weinreb RN. Correlation among choroidal, parapapillary, and retrobulbar vascular parameters in glaucoma. Am J Ophthalmol. 2009;147(4):736-43.
3. Leske MC, Heijl A, Hyman L, Bengtsson B, Dong L, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114(11):1965-72.
4. Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115(1):85-93.
5. Maruko I, Iida T, Sugano Y, Ojima A, Sekiryu T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31(8):1603-8.
6. Nakayama M, Keino H, Okada AA, et al. Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina;32(10):2061-9.
7. Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445-50.
8. Manjunath V, Goren J, Fujimoto JG, Duker JS. Analysis of choroidal thickness in age-related macular degeneration using spectral-domain optical coherence tomography. Am J Ophthalmology. 2011; 152(4):663-8.
9. Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147(5):811-5.
10. Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011; 118(10):2001-7.
11. Lee EJ, Kim TW, Weinreb RN, Park KH, Kim SH, Kim DM. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011;152(1):87-95.
12. Lee EJ, Kim TW, Weinreb RN, Suh MH, Kang M, Park KH, et al. Three-dimensional evaluation of the lamina cribrosa using spectral- domain optical coherence tomography in glaucoma. Invest Ophthalmol Vis Sci. 2012;53(1):198-204.
13. Rhodes LA, Huisingh C, Johnstone J, Fazio MA, Smith B, Wang L. Peripapillary choroidal thickness variation with age and race in normal eyes. Invest Ophthalmol Vis Sci. 2015;56(3):1872-9.
14. Dursun A, Ozec AV, Dogan O, Dursun FG, Toker MI, Topalkara A, et al. Evaluation of choroidal thickness in patients with pseudoexfoliation syndrome and pseudoexfoliation glaucoma. J Ophthalmol. 2016;2016:3545180.
15. Lin Z, Huang S, Xie B, Zhong Y. Peripapillary choroidal thickness and open-angle glaucoma: a meta-analysis. J Ophthalmol. 2015; 2015: 919610.
16. Hosseini H, Nilforushan N, Moghimi S, Bitrian E, Riddle J, Lee GY. Peripapillary and macular choroidal thickness in glaucoma. J Ophthalmic Vis Res. 2014;9(2):154-61.
17. Chakraborty R, Read SA, Collins MJ. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 2011;52(8):5121-9.
18. Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol. 2009;148(3):445-50.
19. Dayanir V, Topaloğlu A, Ozsunar Y, Keceli M, Okyay P, Harris A. Orbital blood flow parameters in unilateral pseudoexfoliation syndrome. Int Ophthalmol. 2009;29(1):27-32.
20. Kantarci FA, Tatar MG, Uslu H, Colak HN, Yildirim A, Goker H, Gurler B. Choroidal and peripapillary retinal nerve fiber layer thickness in adults with anisometropic amblyopia. Eur J Ophthalmol. 2015;25(5):437-42.
21. Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53(1):261-6.
Submitted for publication:
June 23, 2017.
Accepted for publication:
January 14, 2018.
Approved by the following research ethics committee: Sisli Hamidiye Etfal Training and Research Hospital (# 1347/2016)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose