

Yusuf Koçluk1; Burcu Kasim1; Emine Alyamaç Sukgen1; Ayşe Burcu2
DOI: 10.5935/0004-2749.20180043
ABSTRACT
Purpose: To evaluate the complications and clinical results of Descemet membrane endothelial keratoplasty (DMEK) in patients with endothelial failure that occurred during the learning curve of a surgeon.
Methods: Fifty eyes of 50 patients with DMEK and ≥6 months of follow-up were included. The patients were divided into the first 25 (group 1) and the second 25 (group 2) procedures performed by the surgeon. Best corrected visual acuity (BCVA), central corneal thickness (CCT), unfolding time of the Descemet membrane (DM) graft, and intraoperative and postoperative complications were compared between groups.
Results: The differences in postoperative increase of BCVA (p=0.595) and decrease of CCT (p=0.725) in the two groups were not significant. The unfolding time of the DM was longer in group 1 than in group 2 (p=0.001). Primary graft failure occurred in three patients in group 1 and none in group 2. At the last visit, 42 (85.7%) of patients' corneas were clear, with significant difference between groups (p=0.584). A patient in group 1 with a history of pars plana vitrectomy, inferior iridectomy, and fluid as a tamponade experienced drop of the DM graft into the iridectomy space. All other intraoperative complications occurred in group 1.
Conclusions: Occurrence of intraoperative and postoperative complications was increased in patients with coexisting ocular pathology or complicated endothelial dysfunction and during the surgeon's learning curve of DM endothelial keratoplasty procedures.
Keywords: Descemet membrane; Corneal disease/surgery; Descemet stripping endothelial keratoplasty; Intraoperative complications; Postoperative complications
RESUMO
Objetivo: Avaliar as complicações e os resultados clínicos da queratoplastia endotelial da membrana de Descemet (DMEK) em indivíduos com insuficiência endotelial, durante a curva de aprendizado de um cirurgião.
Métodos: Cinquenta olhos de 50 pacientes submetidos ao procedimento queratoplastia endotelial da membrana de Descemet com pelo menos 6 meses de acompanhamento foram incluídos neste estudo. Os pacientes foram divididos em dois grupos: como os primeiros 25 casos do cirurgião (grupo 1) e como os 25 casos seguintes (grupo 2). A melhor acuidade visual corrigida (MAVC), a espessura corneana central (ECC), o tempo de desdobramento do enxerto da membrana de Descemet (MD), as complicações intraoperatórias e pós-operatórias foram apresentadas e comparadas entre os grupos.
Resultados: Os grupos não diferiram estatisticamente em relação ao aumento pós-operatório de melhor acuidade visual corrigida (p=0,595) ou à diminuição da espessura corneana central (p=0,725). O tempo de desdobramento dos enxertos de membrana de Descemet no grupo 1 foi maior do que no grupo 2 (p=0,001). Falha do enxerto primário foi observada em 3 pacientes do grupo 1 e em nenhum do grupo 2. Na última visita, 42 (85,7%) das córneas dos pacientes estavam claras e não foram observadas diferenças estatisticamente significativas entre os grupos (p=0,584). Na cirurgia de um paciente do grupo 1, com história de vitrectomia pars plana (PPV) com iridectomia inferior e fluido como tamponamento, observou-se queda do enxerto de membrana de Descemet no local da iridectomia. Além disso, todas as demais complicações intraoperatórias ocorreram no grupo 1.
Conclusões: As complicações intraoperatórias e pós-operatórias foram maiores em pacientes com coexistência de outra patologia ocular ou com disfunção endotelial complicada durante as curvas de aprendizado dos cirurgiões no procedimento queratoplastia endotelial da membrana de Descemet.
Descritores: Lâmina limitante posterior; Doenças da córnea/cirurgia; Ceratoplastia endotelial com remoção da lâmina limitante posterior; Complicações intraoperatórias; Complicações pós-operatórias
INTRODUCTION
Descemet membrane endothelial keratoplasty (DMEK) has faster visual rehabilitation, lower rejection risk and better anatomical repair with less tissue transplantation than other endothelial keratoplasty (EK) techniques(1-3). A stromal layer with endothelium and Descemet membrane (DM) is transplanted in alternative EK procedures, but only endothelium and the DM is replaced in DMEK. DMEK was first described by Melles et al. in 2006(4). The procedure requires specific training in surgical technique and tissue handling(5). It is effective for the treatment of endothelial decompensation in complex preoperative situations, but the vision outcomes, rebubbling, and graft failure are more frequent compared with DMEK under similar circumstances(6). Fuchs' endothelial dystrophy, pseudophakic bulluos keratopathy (PBK), DMEK is indicated for posterior polymorphous dystrophy, congenital hereditary endothelial dystrophy, iridocorneal endothelial syndrome, endothelial failure for any reason in which the stroma is intact, and endothelial failure after penetrating keratoplasty (PKP)(7). The aim of this study was to evaluate the intraoperative and postoperative complications and clinical results of consecutive DMEK surgeries in patients with endothelial failure without stromal opacification.
METHODS
Fifty eyes of 50 patients with DMEK and ≥6 months of follow-up at the ophthalmology clinic of a training and research hospital between February 2014 and February 2016 were included in this retrospective study. The study was approved by the local ethics review board and followed the ethical principles of the Declaration of Helsinki. Demographic characteristics; slit-lamp biomicroscopy findings; best corrected visual acuity (BCVA) using the logarithm of the minimum angle of resolution (logMAR); intraocular pressure (IOP) using Goldmann applanation tonometry; corneal transparency (clear, no stromal haze, and a clear view of anterior segment structures; edematous, corneal edema with stromal haze obscuring a detailed view of the iris or pupil); DM graft attachment; central corneal thickness (CCT) preoperatively and on postoperative day 1, month 1, and last follow-up visit; and intraoperative findings and complications were recorded and evaluated. The DM graft unfolding time in the anterior chamber, interval between insertion of the graft into the anterior chamber and full unfolding in the correct orientation were measured in surgical videos. DM unfolding time was stratified as 0-10, 10-20, and >20 min. Graft unfolding time, intraoperative, postoperative complications, and other results in the first 25 (group 1) and the second 25 (group 2) were compared. Patients with incomplete follow-up or less than 6 months of follow-up were excluded.
Surgical procedure
Donor graft preparation: Donor tissues were obtained from the hospital eye bank. For DM grafts, corneas from donors between 55 and 70 years of age were preferred. Donor tissues with conditions that threatened the endothelium, such as endothelial folds, guttata, or pigment deposition on microscopic evaluation, or a history of previous intraocular surgery were not used as DM grafts. Endothelial cell counts could not be performed because of the absence of donor specular microscopy in our department. Corneal DM grafts were evaluated by biomicroscopy before transplantation and were prepared in the operating room immediately before surgery. Graft diameter of graft was measured from limbus to limbus of the recipient eye. All grafts were prepared using the submerged corneas using backgrounds away technique as previously described(8). Donor corneas were placed on a vacuum punch (Katena Products, Inc., New Jersey, USA), a 0.06%. Trypan blue solution was used to stain the edges of DM. Under balanced salt solution (BSS), a liftable edge was created using a Sinskey hook anterior to Schwalbe's line, and the circumference of the DM was separated. A free area of DM edge was grasped with tying forceps and peeled away from the stroma. When nearly half of the DM was stripped, the graft was restained and replaced over the stroma. Superficial partial thickness trephination was performed from the endothelial side with the previously determined diameter, and the DM stripping then was completed under BSS. The donor graft was restained with 0.06% trypan blue and aspirated into a glass injector system (DMEK Surgical Disposable Set, INNOVA Medical Ophthalmics, Toronto, Canada) in fluid for delivery into the anterior chamber of the recipient eye.
Recipient eye preparation, graft insertion, and graft unfolding and positioning: All Procedures were performed under sub-tenon anesthesia by a single surgeon (Y.K.). The corneal epithelium was removed to enhance visualization of the anterior chamber. Clear corneal incisions were made from the temporal, nasal, and superior quadrants with a 23-gauge knife. The anterior chamber was filled with air, and the DM of the recipient eye, with a diameter approximately 0.5 mm larger than the DM graft, was stripped 360° with a reverse sinskey hook and removed from the anterior chamber. The DM was scraped as large as previous grafts in PKP patients. All patients underwent an intraoperative inferior peripheral iridectomy before DM insertion. In four patients, combined phacoemulsification and intraocular lens implantation (Phaco-IOL) was performed before any steps of the DMEK procedure. The superior corneal incision was enlarged to 2.8 mm, and the rolled DM graft was inserted into the anterior chamber using the glass injector system. At this point, the anterior chamber was shallow, and the eye was soft. The main incision was closed with a 10/0 nylon suture. If the tissue was tightly rolled up, a cannula was placed anterior to the graft. If the tip did find the roll and remained silver, then a quick flush of BSS was given to roll the graft to the correct orientation. If the tip could be positioned under the roll and turned blue, the graft was oriented correctly, that is, blue cannula or Moutsouris sign, and no additional marking was used to confirm the graft orientation(9). A tapping technique together with intracameral short bursts of fluid was used to unfold and position the graft with the endothelium on the outer side(10). The anterior chamber was then filled with air. Many different graft unfolding techniques have been described, but all follow a stepwise approach to unfolding a double scroll by tapping the cornea in a shallow anterior chamber and the use of an air bubble to assist working with tight or single scrolls(11). At the end of the procedure, a therapeutic contact lens was placed on the corneal surface, the patient was moved to the recovery room and kept supine for 2 h, and was discharged home and instructed to remain supine for 48 h. All patients received standard postoperative topical corticosteroids and antibiotics.
Statistical analysis
Statistical analysis was performed with SPSS, version 16.0 for Windows (SPSS, Inc., Chicago, 1L, USA). Data that were found to be normally distributed by the Kolmogorov-Simirnov test were reported as means ± standard deviation. Where appropriate, independent sample t-tests and paired sample t-test were used. Categorical variables were reported as frequency (%) and compared using chi-square and Fisher's exact tests. Statistical significance was determined as p<0.05.
RESULTS
Fifty eyes of 50 patients with DMEK surgery were divided into the surgeon's first 25 (group 1, 10 men and 15 women) and second 25 (group 2, 9 men and 16 women) procedures. Demographics, preoperative diagnosis, ocular comorbidities, and other clinical characteristics are shown in table 1. Seven patients, three in group 1 and four in group 2, had glaucoma with or without either cataracts or optic atrophy. All glaucoma patients were medically controlled and had IOP <21 mmHg prior to surgery. The DM graft unfolding times were <10 min in three (12%) patients in group 1 and 16 (64%) in group 2, between 10 and 20 min in 14 (58%) patients in group 1 and six (24%) in group 2, and >20 min in seven (29.2%) patients in group 1 and three (12%) in group 2 (all p= 0.001, Table 2).
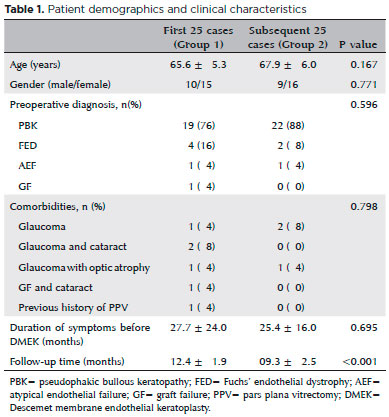
Intraoperative complications
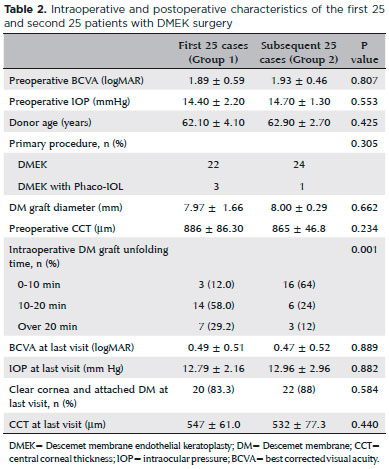
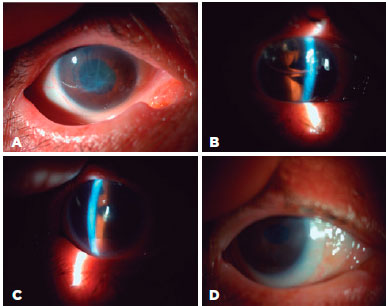
All intraoperative complications occurred in group 1 One patient with a history of pars plan vitrectomy (PPV) for a dropped nucleus with inferior iridectomy and fluid tamponade experienced a drop of the DM graft through the iridectomy space. The dropped DM was brought back into the anterior chamber through the iridectomy space with an infusion cannula inserted through the pars plana. As it was thought that the patient was unsuitable for DMEK, a PKP was performed in the same session (Figure 1). DMEK with Phaco-IOL was performed in another group 1 patient with a PKP
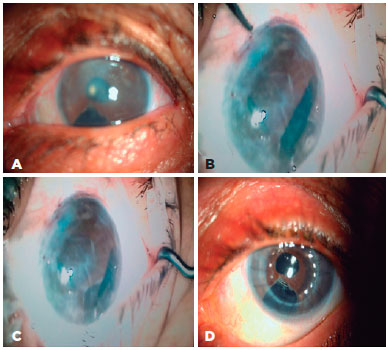
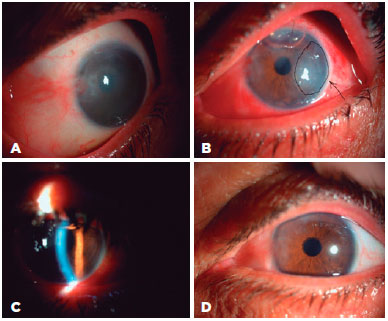
2 years previously. During the procedure, the peripheral cornea and the details of anterior chamber could not be visualized clearly because of opacifications between the donor and recipient corneas. For that reason, DM graft unfolding was prolonged. Despite DM graft attachment, postoperative corneal edema persisted. Three months after surgery, re-PKP was performed with successful achievement of graft transparency (Figure 2). Two group 1 patients with glaucoma, cataracts, and endothelial failure who underwent DMEK with Phaco-IOL experienced prolonged DM graft unfolding because of constant anterior chamber shallowing. Both patients had normal preoperative IOP with medication. The DM grafts eventually attached to stromal side with air, but postoperative corneal edema persisted. Re-DMEK was planned for both patients (Figure 3).
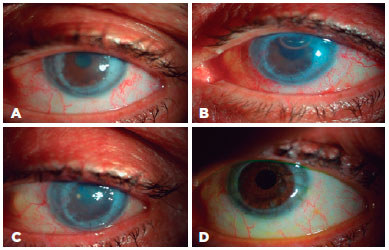
Clinical results and postoperative complications
The postoperative BCVA was significantly improved at each follow-up visit compared with preoperative acuity (p<0.001, for all three comparisons), and the improvements in groups 1 and 2 were not significantly different (p= 0.595). The postoperative decreases in IOP that were seen at all three postoperative visits were significant (p<0.001, for all three comparisons). The differences of IOP that occurred in groups 1 and 2 were not statistically significant (p=0.892). The mean preoperative corneal apex pachymetry values were 886 ± 86.3 µm in group 1 and 865±46.8 in group 2 (p=0.234). The values at the last postoperative visit were 547 ± 61.0 µm in group 1 and 532 ± 77.3 in group 2 (p=0.440). The decrease of corneal thickness was statistically significant within both groups (p<0.001), but not between groups (p=0.725). Preoperative and postoperative clinical results and between-group comparisons are shown in table 2.
Forty-one patients (83.7%) had clear corneas on the first day after surgery. The corneas were clear in 43 (87.8%) patients at 1 month, and in 42 (85.7%) at the last follow-up visit. On day 1 after surgery, 45 of the DM grafts (91.8%) were attached; peripheral DM detachment was observed in four (8.2%) patients. At 1 month and at the last visit, 49 (98%) of DM grafts were found to be attached. The between-group difference was not significant (p= 0.584). Two patients in group 1 and two in group 2 with DMEK for PBK experienced decentralization of the peripheral DM graft onto the recipient DM with roll formation at 1 week after surgery because of an intraoperative decentered DM graft. However, the DM graft was attached to the central cornea, which was clear. Recipient DM scraping with rebubbling was performed at postoperative week 1 in these patients (4% each in groups 1 and 2) and DM attachment and corneal transparency were obtained in all quadrants (Figure 4). Primary graft failure was seen in three patients (12%) in group 1, one with graft failure after PKP and two with glaucoma. In all three, the DM graft unfolding times had been >20 min. No primary graft failures (0%) occurred in group 2 (0%). Secondary surgeries were performed in 16% of the patients. In group 1, these were PKP in one patient (4%) at the same session and postoperatively in another (4%) patient. In group 2, secondary surgery was for PKP with glaucoma in one patient (4%). Re-DMEK was performed in four (16%) patients, two in each group. In the group 2 patients, the corneas were clear on both day 1 and month 1. Consequently, secondary graft failure was considered. The rates of secondary surgeries (16% in group 1 vs. 12% in group 2 were not significantly different (p= 0.388).
DISCUSSION
DMEK offers better visual potential, faster rehabilitation, and lower rejection risk than PKP and other posterior keratoplasties with DM(10). It can be used in different endothelial pathologies and is also possible in complicated cases such as failed penetrating grafts or eyes with anterior chamber, scleral-sutured or iris-sutured intraocular lenses(11-14). A triple procedure including DMEK combined with cataract surgery and IOL implantation is an effective and safe treatment choice for patients with endothelial dystrophy and cataracts(15). DMEK can also successfully restore visual acuity even in vitrectomized eyes, but the overall complication rate in such cases was found to be higher than that in standard DMEK surgery(16). In vitrectomized eyes, the unfolding process was prolonged, included iatrogenic intraocular damage to some extent, and unpredictable maneuvers had to be performed in the anterior chamber(16). In this study, the procedure in a vitrectomized eye was switched to PKP because of the drop of the DM graft. As far as we know, the drop of DM graft during a DMEK procedure has not been previously reported. That patient with fluid in the vitreous space had an iridectomy and an IOL placed in the sulcus. Fluid flow was observed between anterior and posterior chambers during the procedure. The dropped DM was removed to the anterior chamber through the same iridectomy space using an infusion cannula inserted through the pars plana. However, the injected air bubble that attached the DM to the stroma also passed through the same space to posterior chamber. A different donor cornea was used to perform PKP during the same session. DMEK can be expected to be a complicated procedure in patients with previous PPV and iridectomy. This patient was one of the first 25 cases of the surgeon and might have been handled better with more experience. Several vitrectomized patients were treated during the preparation of this report, but were not included because of the short follow-up that would have been possible. Those surgeries resulted in successful DMEK without intraoperative complications. However, DM unfolding times were prolonged. Considering the DMEK learning curve, uncomplicated cases should be selected.
DMEK can be considered to treat failed PKP, but postoperative peripheral DM graft detachment and need for reinjection of air have been reported(12). Graft unfolding was prolonged in a patient with a cataract and graft failure who underwent DMEK with Phaco-IOL, and iatrogenic DM graft failure required postoperative re-PKP. In two patients with glaucoma and endothelial failure, DM graft unfolding time was over 20 min because of a shallow anterior chamber and iris prolapsus from corneal incisions. Iatrogenic DM graft failure was also considered in these patients with persistent postoperative corneal edema, and re-DMEK was proposed to both patients. In a case report, DMEK appeared feasible in the management of corneal endothelial decompensation in eyes with glaucoma implants. The authors caution that the DM graft, IOP, and endothelial cell density be closely monitored(17).
A previous study, compared the outcomes of “early surgeries” performed during transition to a standard technique with those of “late surgeries” after technique standardization(18). Standardization may have contributed to a decrease in graft detachment and a relatively low secondary intervention rate. Postoperative complications decreased from 23.2% to 10% and the need for secondary surgery decreased from 6.8% to 3.6% of cases(18). In this study, unfolding time of the DM graft in the anterior chamber was significantly longer in the first group of cases than in the second group. The unfolding time decreased as the surgeon standardized the technique and gained experience. Another study found that intraoperative complications were rare, with infrequent difficulties in inserting, unfolding, or positioning of the graft (1.2%) and intraoperative hemorrhage (0.5%)(19). Graft detachment (34.6%) was the most frequent postoperative complication in that study, and 20.4% were managed with a single rebubbling procedure, but a second (2.6%) or third rebubbling (0.7%) was needed, and 17.6% of the patients experienced a second keratoplasty(19). The search for novel techniques to improve surgery for EK is ongoing(20,21). In this study, secondary surgeries were performed in 16% of cases. Peripheral graft detachment that required postoperative rebubbling occurred in 8.2% of the cases. Because grafts from older donors generally scroll less tightly, presumably because the DM thickens with age(22), donors over 40 years of age were preferred for greater ease of graft unscrolling. The success rate of donor preparation using preferred techniques is currently over 99%(23). In this study, the cornea donors were from 55 to 70 years of age; donor preparation were successful in all cases.
A study limitation was that endothelial cell count could not be performed at preoperative and postoperative stages. Despite the reporting of endothelial cell counts in most previous studies, this evaluation has not been considered essential. In this study, postoperative biomicroscopic assessment of the corneas found that 85.7% of the DM grafts were clear. Other limitations were relatively short follow-up and a limited number of cases. Also, corneal densitometry could have been used as an objective evaluation of corneal transparency instead of subjective biomicroscopic findings.
In conclusion, intraoperative and postoperative complications may be increased in patients with coexisting ocular pathology and in procedures performed by surgeons during the DMEK learning curve. The unfolding time of DM graft may decrease as the surgeon gains experience.
REFERENCES
1. Guerra FP, Anshu A, Price MO, Price FW. Endothelial keratoplasty: fellow eyes comparison of Descemet stripping automated endothelial keratoplasty and Descemet membrane endothelial keratoplasty. Cornea. 2011;30(12):1382-6.
2. Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118(12):2368-73.
3. Tourtas T, Laaser K, Bachmann BO, Cursiefen C, Kruse FE. Descemet membrane endothelial keratoplasty versus Descemet stripping automated endothelial keratoplasty. Am J Ophthalmol. 2012; 153(6):1082-90.
4. Melles GR. Posterior lamellar keratoplasty: DLEK to DSEK to DMEK. Cornea. 2006;25(8):879-81. Comment in: Cornea. 2006;25(8): 886-9.
5. Ham L, Dapena I, van Luijk C, van der Wees J, Melles GR. Descemet membrane endothelial keratoplasty (DMEK) for Fuchs endothelial dystrophy: review of the first 50 consecutive cases. Eye (Lond). 2009;23(10):1990-8.
6. Weller JM, Tourtas T, Kruse FE. Feasibility and outcome of Descemet membrane endothelial keratoplasty in complex anterior segment and vitreous disease. Cornea. 2015;34(11):1351-7.
7. Feng MT, Price MO, Price FW. Update on Descemet membrane endothelial keratoplasty (DMEK). Int Ophthalmol Clin. 2013; 53(2):31-45.
8. Stuart A. Performing DMEK: A Step-By-Step Guide. Eyenet. 2014; 23-25. [cited 2016 Jul 21]. Available at: https://www.aao.org/eyenet/article/performing-dmek-stepbystep-guide.
9. Price MO, Giebel AW, Fairchild KM, Price FW Jr. Descemet’s membrane endothelial keratoplasty: prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology. 2009;116(12):2361-8. Comment in: Ophthalmology. 2010;117(7):1459-60.
10. Yoeruek E, Bayyoud T, Hofmann J, Bartz-Schmidt KU. Novel maneuver facilitating Descemet membrane unfolding in the anterior chamber. Cornea. 2013;32(3):370-3.
11. Liarakos VS, Dapena I, Ham L, van Dijk K, Melles GR. Intraocular graft unfolding techniques in Descemet membrane endothelial keratoplasty. JAMA Ophthalmol. 2013;131(1):29-35. Comment in: JAMA Ophthalmol. 2013;131(1):88-9.
12. Anshu A, Price MO, Price FW Jr. Descemet membrane endothelial keratoplasty and hybrid techniques for managing failed penetrating grafts. Cornea. 2013;32(1):1-4.
13. Liarakos VS, Ham L, Dapena I, Tong CM, Quilendrino R, Yeh RY, et al. Endothelial keratoplasty for bullous keratopathy in eyes with an anterior chamber intraocular lens. J Cataract Refract Surg. 2013;39(12):1835-45.
14. Rock D, Rock T, Bartz-Schmidt KU, Yoeruek E. Descemet membrane endothelial keratoplasty in cases with existing scleral-sutured and iris-sutured intraocular lenses. BMC Ophthalmol. 2014;14:6.
15. Chaurasia S, Price FW Jr, Gunderson L, Price MO. Descemet’s membrane endothelial keratoplasty: clinical results of single versus triple procedures (combined with cataract surgery). Ophthalmology. 2014;121(2):454-8.
16. Yoeruek E, Rubino G, Bayyoud T, Bartz-Schmidt KU. Descemet membrane endothelial keratoplasty in vitrectomized eyes: clinical results. Cornea. 2015;34(1):1-5.
17. Heindl LM, Koch KR, Bucher F, Hos D, Steven P, Koch HR, et al. Descemet membrane endothelial keratoplasty in eyes with glaucoma implants. Optom Vis Sci. 2013;90(9):241-4; discussion 1029.
18. Rodríguez-Calvo-de-Mora M, Quilendrino R, Ham L, Liarakos VS, van Dijk K, Baydoun L, et al. Clinical outcome of 500 consecutive cases undergoing Descemet’s membrane endothelial keratoplasty. Ophthalmology. 2015;122(3):464-70.
19. Monnereau C, Quilendrino R, Dapena I, Liarakos VS, Alfonso JF, Arnalich-Montiel F, et al. Multicenter study of descemet membrane endothelial keratoplasty: first case series of 18 surgeons. JAMA Ophthalmol. 2014;132(10):1192-8. Comment in: JAMA Ophthalmol. 2015;133(6):725. JAMA Ophthalmol. 2015;133(6):724-5.
20. Steverink JG, Wisse RP. Intraoperative optical coherence tomography in descemet stripping automated endothelial keratoplasty: pilot experiences. Int Ophthalmol. 2017;37(4):939-44.
21. Titiyal JS, Tinwala SI, Shekhar H, Sinha R. Sutureless clear corneal DSAEK with a modified approach for preventing pupillary block and graft dislocation: case series with retrospective comparative analysis. Int Ophthalmol. 2015;35(2):233-40.
22. Heindl LM, Hofmann-Rummelt C, Schlotzer-Schrehardt U, Kruse FE, Cursiefen C. Histologic analysis of Descemet stripping in posterior lamellar keratoplasty. Arch Ophthalmol. 2008;126(4):461-4. Comment in: Arch Ophthalmol. 2009;127(2):225-6; author reply 226-7.
23. Guerra FP, Anshu A, Price MO, Giebel AW, Price FW. Descemet’s membrane endothelial keratoplasty: prospective study of 1-year visual outcomes, graft survival, and endothelial cell loss. Ophthalmology. 2011;118(12):2368-73.
Submitted for publication:
January 11, 2017.
Accepted for publication:
December 19, 2017.
Approved by the following research ethics committee: Girişimsel Olmayan Klinik Araştırmalar (# 209/2015)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose