

Diva Vargas1; Cleudson Castro2,3
DOI: 10.5935/0004-2749.20180041
ABSTRACT
Purpose: We investigated parasympathetic innervation abnormalities of the iris sphincter and ciliary muscles in chronic Chagas disease by measuring pupillary diameter and intraocular pressure.
Methods: A group of 80 patients with Chagas disease was compared with 76 healthy individuals without chagasic infection. The following procedures were performed: pupillometry, hypersensitivity test to pilocarpine 0.125%, intraocular pressure measurement (IOP), basal pupil diameter (BPD), absolute pupillary constriction amplitude (ACA), relative pupillary constriction amplitude (RCA) and the presence of anisocoria.
Results: The prevalence of anisocoria was higher in chagasic patients (p<0.01). These patients had mean basal pupillary diameter, mean photopic pupillary diameter and mean value of absolute pupillary constriction amplitude significantly lower than non-chagasic ones (p<0.01, mean difference -0.50mm), (p=0.02, mean difference -0.20mm), (p<0.01, mean difference -0.29mm), respectively. The relative pupillary constriction amplitude did not differ between the two groups (p=0.39, mean difference -1.15%). There was hypersensitivity to dilute pilocarpine in 8 (10%) of the chagasic patients in the right eye and in 2 (2.5%) in the left eye and in 1 (1.25%) in both eyes. The mean value of intraocular pressure had a marginal statistical significance between the two groups (p=0.06, mean difference -0.91mmHg).
Conclusions: Patients with chagasic infection may exhibit ocular parasympathetic dysfunction, demonstrable by pupillometry and the dilute pilocarpine hypersensitivity test.
Keywords: Pupil physiology; Parasymphatetic denervation; Hypersensitivity; Intraocular pressure; Pilocarpine; Chagas disease
RESUMO
Introdução: Investigaram-se anormalidades da inervação parassimpática dos músculos esfíncter da íris e ciliar na doença de Chagas crônica, através de medidas pupilares e da pressão intraocular.
Métodos: Foram estudados dois grupos, um com 80 chagásicos e outro com 76 indivíduos saudáveis sem infecção chagásica. Foram realizados os seguintes procedimentos: pupilometria, teste de hipersensibilidade à pilocarpina a 0,125%, medida da pressão intraocular (PIO), diâmetro basal da pupila (DBP), amplitude de constrição pupilar absoluta (ACA), amplitude de constrição pupilar relativa (ACR), e presença de anisocoria.
Resultados: A prevalência de anisocoria foi maior nos chagásicos (p<0,01). Estes pacientes apresentaram diâmetro basal pupilar médio, diâmetro fotópico médio e valor médio da amplitude de constrição pupilar absoluta, significativamente menores que os não chagásicos, (p<0,01, diferença de média -0,50mm), (p=0.02, diferença de média -0,20mm), (p<0,01, diferença de média -0,29mm), respectivamente. A amplitude de constrição pupilar relativa não diferiu entre os dois grupos (p=0,39, diferença de média -1,15%). Houve hipersensibilidade à pilocarpina diluída em 8 (10%) chagásicos no olho direito em 2 (2,5%) no olho esquerdo e em 1 (1,25%) em ambos os olhos. O valor médio da pressão intraocular teve significância marginal entre os dois grupos (p=0,06, diferença de média -0,91mmHg).
Conclusões: Pacientes com infecção chagásica podem apresentar disfunção parassimpática ocular, demonstrável pela pupilometria e pelo teste de hipersensibilidade à pilocarpina diluída.
Descritores: Fisiología da pupila; Parassimpatectomia; Hipersensibilidade; Pressão intraocular; Pilocarpina; Doença de Chagas
INTRODUCTION
Damage to the parasympathetic pathway of the autonomic nervous system (ANS) is a hallmark of Chagas disease. Previous studies of patients with chronic chagasic infection have demonstrated anisocoria and abnormal pupillary reactivity to diluted pilocarpine(1,2). There are reports of lower intraocular pressure (IOP) in these patients as compared with controls(3) and an orthostatic drop in IOP(4), suggesting that the ANS regulates the pressure. IOP represents the balance between production and drainage of aqueous humor and is controlled by autonomic neurons that regulate ocular blood flow. The sympathetic nerves regulate production by controlling blood vessels in the ciliary body epithelium, and the parasympathetic nerves regulate the trabecular meshwork and episcleral blood vessels to control drainage of aqueous humor(5).
The pupil diameter is also controlled by sympathetic and parasympathetic balance. Parasympathetic activation causes miosis by sphincter iris muscle contraction, and sympathetic stimulation causes mydriasis by radial muscle contraction. The pupil is frequently affected in generalized autonomic neuropathies(6). Functional iris damage is often observed in diseases with parasympathetic deprivation such as diabetes mellitus(7) and uremia(8). The pupil's response to light is thus an indirect way of evaluating the integrity of the ANS pathways that control its diameter, as long as the afferent sensory pathway is preserved(9). Pupillary diameter is regarded as a sensitive and reliable measure to evaluate the ANS(10). Infrared pupillometry can assess pupil diameter and detect iris sphincter hypersensitivity. The latter involves measuring pupillary constriction before and after application of pilocarpine 0.125% to assess parasympathetic denervation. Iris sphincter hypersensitivity to dilute parasympathomimetic agents has been demonstrated as a good way of testing for early autonomic dysfunction in diabetes(11).
METHODS
This was a nonrandomized, cross-sectional study approved by The Research Ethics Committee of the Faculty of Medicine of the University of Brasília. The study adhered to the tenets of the Declaration of Helsinki.
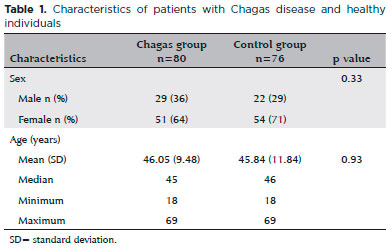
Two groups of participants were recruited from our outpatient clinic. The Chagas group comprised 80 patients (29 men, 51 women) diagnosed with Chagas disease based on results of either one positive xenodiagnosis or two positive serology tests. A control group included 76 individuals (22 men, 54 women) with at least one negative serology result for Chagas disease. Patients in the Chagas group had long-term infection but no heart failure or major arrhythmia, although some had mild electrocardiographic abnormalities and/or megaesophagus. Individuals were excluded from the study if they used medications or had a disease that might interfere with the pupil diameter, pupil reactivity, or IOP. Those with a history of previous ocular trauma or intraocular surgery were also excluded. The mean age of the two groups did not differ significantly (control group, 45.84 ± 11.84 years [range 18-69] vs. Chagas group 46.05 ± 9.48 years [range 18-69]; p = 0.93) (Table 1).
We studied 160 eyes in the Chagas group and 152 eyes in the control group. Participants were asked to abstain from caffeine or other stimulants for 12 hours prior to the examination. The study was performed in a darkened room with light-proof windows maintained at 20°C. For pupillometry under scotopic conditions, the lights were switched off and the door was partially opened to allow sufficient light input for a room luminance of ≤1 lux. The pupil basal diameter (defined as the greatest mydriasis in the dark), the smallest pupillary diameter after light stimulation (maximum miosis), and the absolute constriction amplitude (the difference between mydriasis and miosis) were measured in mm. Finally, the relative constriction amplitude (RCA) was calculated, that is, the ratio between the absolute constriction amplitude and the greatest mydriasis expressed as a percentage. The pupillary diameter was measured with the Colvard monocular pupillometer (Oasis Medical, Inc. Glendora, California, USA). It uses light amplification from low light energy to allow visualization of the pupil. A phosphorescent image is seen through the eyepiece as well as a reticle inside the device with 0.5mm divisions. The largest pupil diameter was measured by recording the reticle marks at the nasal and temporal margins of the horizontal pupil diameter. A periorbital rubber fitting isolated the tested eye, eliminating light and controlling the distance from the pupillometer to the eye.
For the scotopic measurements, the participant remained for 2 minutes in diffuse ambient light (≤1 lux as monitored with a digital lux meter [DM, LX 1010B
HDE, Extech Instruments, Nashua, USA] held close to the face) to allow for retinal rod adaptation. To avoid accommodation miosis, the participant was asked to fix the other eye on a 0.5cm red light point 6 meters ahead. The procedure was repeated in the contralateral eye. Next, the room light was turned on, the pupils were stimulated with a light beam to induce maximum miosis, and the photopic pupillary diameter was measured. This procedure was performed observing the safety standards for radiation applied to the human eye according to the International Commission on Radiation Protection(12). The stimulus was applied 30cm from the eyes with a white LED light of 180cd/m2 luminance (Maglite 4D LED, Mag Instrument, Ontario, Canada). Each eye was tested separately, always testing the right eye first. The measurements were taken in the same office with the same equipment by a single examiner after appropriate training.
The pupillary light, consensual, and accommodation reflexes were evaluated. A slit lamp biomicroscopy examination (Haag Streit Diagnostics, Koeniz, Switzerland) was performed to search for abnormalities suggestive of ocular disease. IOP measurements were performed with a Goldmann applanation tonometer (AT 900, Haag-Streit) coupled to the slit lamp. These measurements were performed in the morning after applying topical anesthesia with proxymetacaine 0.5% (Anestalcon, Alcon, São Paulo, SP, Brazil) and one drop of fluorescein 1% (Fluoresceína, Allergan, Guarulhos, SP, Brazil) to each eye.
The pilocarpine test was performed in all participants, applying two drops of pilocarpine 0.125% at an interval of 5 minutes in both eyes. The diluted pilocarpine was prepared by adding one part of pilocarpine 1% (Pilocarpina, Allergan) to seven parts of physiologic saline solution using strict aseptic criteria. Pupillometry was performed 20 minutes after the last drop was applied. The percentage variation in the pupillary diameter was calculated relative to the basal diameter measured under scotopic conditions and was designated RCAp, indicating the RCA after application of pilocarpine. Assuming that the healthy control participants had normal innervation of the pupillary sphincter muscle, the presence of hypersensitivity to diluted pilocarpine in patients with Chagas disease was assessed by comparing their RCAp values with those of the control group. Hypersensitivity in the Chagas group was defined as an RCAp exceeding the mean RCAp in the control group by more than two standard deviations, based on the criteria of Yamashita et al.(13).
The relative constriction amplitude after pilocarpine (RCAp) in the group without Chagas was calculated according to the formula:

Ethical aspects
All participants signed informed consent. The study was approved by the Ethics Committee of the Faculty of Medicine of the University of Brasilia.
Statistical analysis
The sample size was calculated based on a pilot project. Analyses included descriptive statistics (mean and standard deviation for quantitative variables and frequency for qualitative variables). To verify if the mean values of ocular measurements differed between the two groups and between right and left eyes, a mixed-effects model of analysis of variance for repeated measures was used, with age-adjusted data on individuals nested within the two groups. The prevalence of anisocoria was compared between the groups using the Pearson chi-square test. A p<0.05 was considered significant. The SAS 9.4 program was used for all analysis.
RESULTS
Pupillary reflexes were preserved in all participants. Anisocoria was present in 22 (27.5%) participants with Chagas disease and in 1 (1.32%) participant in the control group (p<0.001).
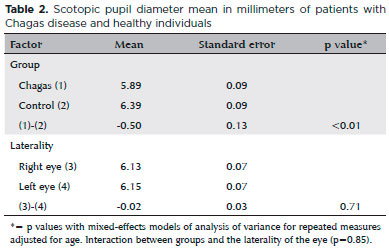
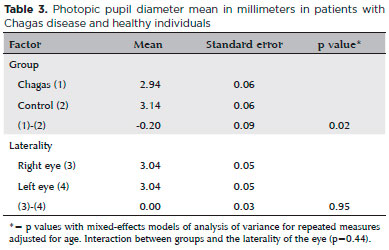
The mean scotopic pupillary diameter, regardless of size, was significantly lower in the Chagas group (p<0.01, mean difference -0.05mm). The mean scotopic pupillary diameter in the right eye was similar to that of the left eye (p=0.71, mean difference -0.02mm) in both groups (Table 2). Similarly, the mean photopic pupillary diameter was lower in the Chagas group, (p=0.02, mean difference -0.20mm) regardless of side. There was no significant difference in the means for the left and right eyes in each group (p=0.95, mean difference 0.00mm) (Table 3). In summary, the mean scotopic and photopic pupillary diameters were significantly lower in patients with Chagas disease.
The mean absolute constriction amplitude was lower in the Chagas group (p<0.01, mean difference -0.29mm), regardless of side. The mean absolute constriction amplitude was similar in both eyes (p=0.77, mean difference -0.01mm) of each group (Table 4). The mean RCA after light stimulus did not differ between the two groups (p=0.39, mean difference -1.15%) and was similar in both eyes (p=0.82, mean difference -0.15%) in each group (Table 5).
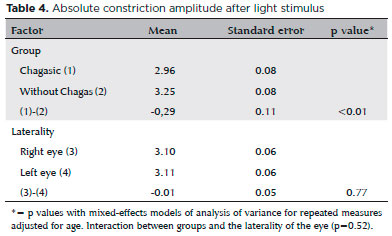
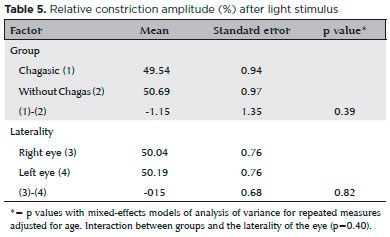
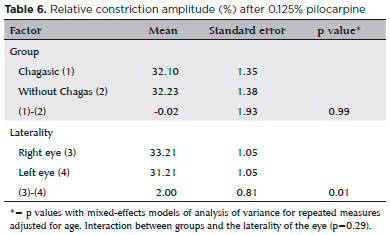
Table 6 shows the relative constriction amplitude after diluted pilocarpine test, whose results were used to investigate hypersensitivity in chagasic patients. As explained in the methodology, the mean relative constriction amplitude after pilocarpine plus two standard deviations, calculated in the control group, was 56.28% in the right eye and 54.93% in the left one - data not shown in the tables. By definition, the results in chagasic patients exceeding the above percentages, in the right and left eye, respectively, were defined as hypersensitivity. Thus, of the 80 chagasic patients, 8 (10%) had hypersensitivity in the right eye, 2 (2.5%) in the left eye and 1 (1.25%) in both eyes.
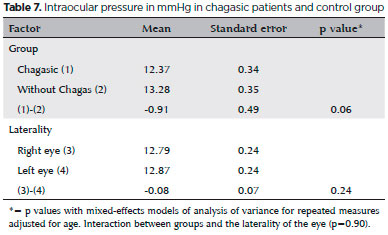
The mean IOP was lower but not significantly so in the Chagas group (p=0.06, mean difference -0.91mmHg). The results were similar for both eyes in each group (p=0.24, mean difference -0.08mmHg) (Table 7).
There was no significant interaction between group and eye laterality for any of the variables.
DISCUSSION
Staying 2 minutes in a dark environment with a luminance of 1 lux is sufficient for stable adaptation of an individual's retina(14). Examination of one eye does not induce accommodation in the other if the unexamined eye stares at a distance. Increasing the duration of the light stimulus from 20 to 240 milliseconds with a fixed light intensity does not change the pupil size(15).
There is no association between the scotopic pupil diameter and the spherical equivalent refraction(16). There is no correlation between pupillary diameter and refractive error, nor are there differences between genders(17). The iris color does not appear to affect the pupillary diameter or its responsiveness(18). The pupil diameter reaches a maximum size between the age of 20 to 29 years old and progressively decreases until the age of 80(14). Thus, in the statistical analysis for this study, adjustment was made only for age.
The threshold for anisocoria varies in the literature. Differences of 1mm and 3mm have been suggested to characterize pupillary asymmetry, neither of which is ideal, since varying the cutoff value changes the specificity and sensitivity(19). Others define anisocoria as a difference equal to or greater than 0.5mm between the pupils(16). Prata et al.(20) used a value equal to or greater than 0.3mm between the pupil diameters. As the Colvard pupillometer reticle has 0.5-mm divisions, we defined anisocoria in this study to be a difference between pupillary diameters equal to or greater than 0.5mm after dark adaptation. We found the prevalence of anisocoria in patients with Chagas disease to be significantly higher (p<0.001) than in the control group. Anisocoria was observed in a previous study in 10 of 131 (7.6%) patients with Chagas and in 3 of 138 (2.1%) controls(1). This lower prevalence as compared to that in our study perhaps reflects differences in methodology. Prata and colleagues defined anisocoria as a difference of 0.3mm and performed pupillometry with a ruler. Even though we used a 0.5-mm difference in pupil size, the digital pupilometer we used had greater sensitivity, including as it did the infrared technology that avoided the need for additional light. A subsequent study by Prata's group(3) using the same 0.3mm cutoff point reported anisocoria in 25 of 84 (29.8%) cases in the Chagas group compared with 10 of 84 (11.9%) in the control group. The anisocoria found in these studies suggests that as Chagas disease affects the ocular ANS, it perhaps affected one eye first, resulting in asymmetry of the pupillary diameter.
The pupil basal diameter was significantly lower in the Chagas group than in the control group. Damage to the parasympathetic pathway along with preserved sympathetic tone could account for this finding. This accords with studies in patients with diabetes who were found to have a smaller pupillary diameter at rest, even before there was other clinical evidence of ANS abnormalities(21,22). Autonomic denervation hypersensitivity of the iris in patients with diabetes could explain the smaller scotopic pupil diameter, even in the presence of an intact sympathetic system; the mechanism may be similar to that observed in the tonic pupil (Adie pupil)(23). It is believed that there is chronic damage to parasympathetic pathways in diabetes, resulting in denervation hypersensitivity(11). The parasympathetic lesion and the hypersensitivity to acetylcholine could explain small pupils in patients with diabetes who have demonstrable parasympathetic denervation. It is possible that the same mechanism explains the smaller basal pupil diameter in the Chagas group in the current study.
Another explanation could be circulating anti-acetylcholine receptor autoantibodies, similar to those identified by Sterin-Borda et al.(24) in patients with chagasic megacolon. These antibodies appear to exert muscarinic-like agonist activity associated with activation of M2 muscarinic receptors and have been associated with cardiac dysautonomia, achalasia, and a hypertonic distal colon. It is possible that the autoantibodies stimulate the iris sphincter muscle, inducing pupillary constriction.
The pupillary photopic and basal diameter were both significantly smaller in the Chagas group compared with the control group. The absolute constriction amplitude was also lower in the Chagas group. However, the RCA did not differ between the two groups.
The hypersensitivity to diluted pilocarpine found in 12.5% of the patients with Chagas disease also suggests dysfunction in some point of the parasympathetic pathway to the iris. This finding is consistent with other reported neurologic complications in chronic Chagas disease. Hypersensitivity is defined as the exaggerated response of a denervated organ to a stimulus normally incapable of generating so great a response. Jacobson noted the formulation of Cannon's Law to describe the behavior of denervated structures. It stated that hypersensitivity could occur because of lesions at any point, preganglionic or postganglionic, in the neuronal pathway to the effector organ. Testing the hypersensitivity of the iris sphincter using dilute pilocarpine is an extension of Cannon's Law applied to the pupil(25). Denervation hypersensitivity can reportedly decrease over time due to reinnervation of the iris sphincter, and it may even disappear in later stages in a tonic pupil(26).
Interestingly, we found that, in response to the pilocarpine test, the mean pupillary diameter was significantly lower in the right eye than in the left eye (p<0.01 mean difference -0.12mm, data not shown) in both groups. We cannot account for this finding. We consistently examined the right eye first, but none of the evaluations without pilocarpine demonstrated a difference in laterality. Bär et al.(10) found a considerably larger pupil diameter in the left eye in healthy individuals, raising the question of hemispheric lateralization of autonomic function, as has been reported in studies of heart rate variation(27,28). It is possible that our finding of laterality in response to the dilute pilocarpine test was related to this putative hemispheric lateralization of autonomic function. Even though we found a statistically significant difference between the right and left eyes of the patients with hypersensitivity, it is probably not clinically relevant. Other studies are needed to clarify the question of cerebral autonomic function laterality.
The mean IOP did not differ significantly between the two groups, although the p value was close to the significance level (p=0.06). Prata et al.(1,3) found a lower IOP in two studies of patients with Chagas disease. Lewallen et al.(29) found a significantly lower mean IOP in patients with leprosy than in controls, suggesting ocular autonomic dysfunction. Autonomic IOP control is more complex than that of iris dynamics. In addition to traditional ANS pathways controlling the regulation of ocular blood flow, aqueous humor production, and IOP, it has been found that afferent trigeminal nerve fibers, once considered only sensory, also influence intraocular blood vessels and smooth muscles. The influence of autonomic neurons on aqueous humor production occurs not only through innervation of the ciliary processes by adrenergic (sympathetic), cholinergic/peptidergic (parasympathetic) and peptidergic sensory neurons (trigeminal neurons), but also through the regulation of blood flow(30). In patients with chronic Chagas disease, an orthostatic decrease in IOP was demonstrated, suggesting autonomic ocular dysfunction and the possible existence of ocular baroreceptors(4). The fact that we did not find a significant difference in the mean IOP in our study may be because the sample size was insufficient to detect a difference.
A possible limitation of the study was the difficulty in making precise measurements with the pupillometer reticle divisions when the edge of the pupil fell between the markings. Errors in reading the reticle may have occurred due to the observer's poor adaptation to darkness, accommodative miosis, or parallax. Other factors that might have influenced the participants' pupillary reactions could include sleepiness, lack of attention, or stress. In addition, there may be a circadian variation in the pupillary diameter. Primary autonomic failure may cause a decrease in lacrimal production which could interfere with the absorption of pilocarpine. We do not know if this decrease in tears is also true in Chagas disease. Corneal permeability and individual variations in pilocarpine penetration of the normal cornea could also interfere with pilocarpine absorption. These possible confounding variables should be controlled for in future studies.
Although it is difficult to draw firm conclusions about the evolution of autonomic dysfunction in Chagas disease from a cross-sectional study, pupillometry can be a marker to identify patients who have ANS cholinergic dysfunction. The pupil hypersensitivity to dilute pilocarpine suggests injury to parasympathetic control of the iris, indicating that Chagas disease may affect the ANS not only in the heart or intestine but also in the eye.
REFERENCES
1. Prata JA, Prata Júnior JA, Castro CN, Macedo V, Prata A. Anisocoria na fase crônica da doença de Chagas. Rev Soc Bras Med Trop. 1995;28(2):131-3.
2. Prata JA. Exame oftalmológico de pacientes com doença de Chagas crônica em Mambaí (GO). Rev Soc Bras Med Trop. 1997; 30(2):169-70.
3. Prata JA, Prata Jr JA, Correia D, Prata AR. Alterações oculares na doença de Chagas crônica: comprovação na região endêmica de Água Comprida (MG). Arq Bras Oftalmol [Internet]. 2000[citado 2016 Maio 11];63(6):445-8. Disponível em: http://www.scielo.br/pdf/abo/v63n6/9607.pdf
4. Luna JD, Sonzini EE, Diaz HD, 1osa DJ, Jufirez CE. Anomalous intraocular pressure changes in Chagas’ disease elicited by postural test. Int Ophthalmol. 1997; 20(6):329-32.
5. Mediero A, Alarma-Estrany P, Pintor J. New treatments for ocular hypertension. Auton Neurosci. 2009;147(1-2):14-9.
6. Bremner F, Smith S. Pupil findings in a consecutive series of 150 patients with generalized autonomic neuropathy. J Neurol Neurosurg Psychiatry. 2006;77(10):1163-8.
7. Datta S, Biswas NR, Saxena R, Deepak KK, Menon V, Garg SP, et al. Ocular and cardiovascular autonomic function in diabetic patients with varying severity of retinopathy. Indian J Physiol Pharmacol. 2005;49(2):171-8.
8. Idiaquez JC, García-Ortez RO, Alonso RK. Parasympathetic denervation of the iris in uremia. Ann Intern Med.1988;109(11):929. Erratum in: Ann Intern Med. 1989;110(7):578.
9. Meeker M, Du R, Bacchetti P, Privitera CM, Larson MD, Holland MC, et al. Pupil examination: validity and clinical utility of an automated pupillometer. J Neurosc Nurs. 2005;37(1):34-40.
10. Bar KJ, Boettger MK, Till S, Dolicek J, Sauer H. Lateralization of pupillary light reflex parameters. Clin Neurophysiol. 2005;116(4):790-8. Comment in: Clin Neurophysiol. 2005;116(10):2505; author reply 2505-6.
11. Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of Diabetes mellitus. Br J Ophthalmol. 2001;85(10): 1225-30. Comment in: Br J Ophthalmol. 2002; 86(11):1319.
12. Sliney D, Aron-Rosa D, DeLori F, Fankhauser F, Landry R, Mainster M, Marshall J, Rassow B, Stuck B, Trokel S, West TM, Wolffe M; International Commission on Non-1onizing Radiation Protection. Adjustment of guidelines for exposure of the eye to optical radiation from ocular instruments: statement from a task group of the International Commission on Non-1onizing Radiation Protection (1CN1RP). Appl Opt. 2005;44(11):2162-76.
13. Yamashita F, Hirayama M, Nakamura T, Takamori M, Hori N, Uchida K, et al. Pupillary autonomic dysfunction in multiple system atrophy and Parkinson’s disease: an assessment by eye-drop tests. Clin Auton Res. 2010;20(3):191-7.
14. Bradley JC, Bentley KC, Mughal A1, Brown SM. Clinical performance of a handheld digital infrared monocular pupillometer for measurement of the dark-adapted pupil diameter. J Cataract Refract Surg. 2010;36(2):277-81.
15. Muppidi S, Adams-Huet B, Tajzoy E, Scribner M, Blazek P, Sapeth EB, et al. Dynamic pupillometry as an autonomic testing tool. Clin Auton Res. 2013;23(6):297-303.
16. Kohnen EM, Zubcov AA, Kohnen T. Scotopic pupil size in a normal pediatric population using infrared pupillometry. Graefe's Arch for Clin Exp Ophthalmol. 2004;242(1):18-23.
17. Hsieh YT, Hu FR. The correlation of pupil size measured by Colvard pupillometer and Orbscan 11. J Refract Surg. 2007;23(8):789-95.
18. Koch DD, Samuelson SW, Haft EA, Merin LM. Pupillary size and responsiveness. 1mplications for selection of a bifocal intraocular lens. Ophthalmology. 1991;98(7):1030-5.
19. Chesnut RM, Gautille T, Blunt BA, Klauber MR, Marshall LE. The localizing value of asymmetry in pupillary size in severe head injury: relation to lesion type and location. Neurosurgery. 1994;34(5):840-6; discussion 845-6.
20. Prata JA, Prata Junior JA, Castro CN, Macedo V, Prata A. [The pupil in the chronic phase of Chagas diseases and the reaction to pilocarpine and phenlephrine]. Rev Soc Bras Med Trop. 1996; 29(6):567-70. Portuguese.
21. Ferrari GL, Marques JL, Gandhi RA, Heller SR, Schneider FK, Tes-faye S, et al. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. 2010;9:26. doi: 10.1186/1475-925X-9-26. Comment in: Auton Neurosci. 2012;167(1-2):4-6.
22. Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002;25(9):1545-50.
23. Wirtschafter JD, Volk CR, Sawchuk RJ. Transaqueous diffusion of acetylcholine to denervated iris sphincter muscle: a mechanism for the tonic pupil syndrome (Adie syndrome). Ann Neurol. 1978; 4(1):1-5.
24. Sterin-Borda L, Goin JC, Bilder CR, 1antorno G, Hernando AC, Borda E. Interaction of human chagasic 1gG with human colon muscarinic acetylcholine receptor: molecular and functional evidence. Gut. 2001;49(5):699-705.
25. Jacobson DM. Cholinergic supersensitivity of the pupil: Has testing for it outlived its diagnostic utility? [Internet]. Presented at: 26th Annual Meeting of North American Neuro-Ophthalmology Society 2000 [cited 2015 Dec 10]. Available from: http://cdmbuntu.lib.utah.edu/utils/getfile/collection/ehsl-nam/id/3818/filename/3862.pdf
26. Koh KM, Kim US. Characteristics of pupillo-accommodative functions according to time of onset, gender and age in tonic pupil. Int J Ophthalmol. 2013;6(5):659-61.
27. Yoon BW, Morillo CA, Cechetto DF, Hachinski V. Cerebral hemispheric lateralization in cardiac autonomic control. Arch Neurol. 1997;54(6):741-4.
28. Zamrini EY, Meador KJ, Loring DW, Nichols FT, Lee GP, Figueroa RE, et al. Unilateral cerebral inactivation produces differential left/ right heart rate responses. Neurology. 1990;40(9):1408-11. Comment in: Neurology. 1991;41(12):2015.
29. Lewallen S, Courtright P, Lee HS. Ocular autonomic dysfunction and intraocular pressure in leprosy. Br J Ophthalmol. 1989;73(12):946-9. Comment in: Br J Ophthalmol. 1989;73(12):939.
30. Neuhuber W, Schrodl. Autonomic control of the eye and the iris. Auton Neurosci. 2011;165(1):67-79.
Submitted for publication:
July 17, 2017.
Accepted for publication:
December 19, 2017.
Approved by the following research ethics committee: Faculty of Medicine of the University of Brasília (#49281115.5.0000.5558)
Funding: No specific financial support was available for this study
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose