

Luzia Diegues Silva1; Albert Santos1; Luciene Barbosa de Sousa1; Norma Allemann1,2; Lauro Augusto de Oliveira1
DOI: 10.5935/0004-2749.20180010
ABSTRACT
Purpose: To report the results of high-resolution anterior segment optical coherence tomography of patients implanted with a type 1 Boston keratoprosthesis (KPro).
Methods: The retrospective study cohort included 11 eyes of 11 patients (average age, 58.4 years; range, 34-83 years). All subjects underwent anterior segment optical coherence tomography at a single posteoperative time point. The main outcome measures were retro-backplate and retro-optic membrane formation, thinning and gap formation of the corneal carrier graft (melting), and degree of angle closure.
Results: Preoperative diagnoses included chemical burn (55%), failed corneal transplant (36%), and Stevens-Johnson syndrome (9%). The mean postoperative follow-up duration was 38.5 (range, 12-72) months. The most frequent findings of anterior segment optical coherence tomography were retroprosthetic membrane formation (63%, 7/11), thinning of the corneal carrier graft (melting; 55%, 6/11), and a narrow or closed angle (91%, 10/11). Other less common findings were epithelial growth over the optic surface and periprosthetic cyst formation. Retroprosthetic membrane formation was observed in all patients with melting (6/11).
Conclusions: Detailed postoperative examination and visualization of subtle changes of keratoprosthesis implanted eyes by slit lamp biomicroscopy are often difficult. Anterior segment optical coherence tomography is a useful, noninvasive, and quantitative imaging technique that provides useful information to postoperatively monitor the anatomic stability of an implanted keratoprosthesis.
Keywords: Keratoprosthesis; Corneal diseases; Visual prosthesis; Optical coherence tomography; Anterior eye segment
RESUMO
Objetivos: Reportar os resultados das imagens de pacientes com Ceratoprótese de Boston tipo I (KPro) usando tomografia de coerência óptica de alta resolução do seguimento anterior (AS-OCT).
Métodos: Nós realizamos um estudo retrospectivo de pacientes submetidos à KPro. Um total de 11 olhos de 11 pacientes foram incluídos. As imagens de AS-OCT foram realizadas em um único tempo de pós-operatório. Os principais resultados incluem formação de membrana retroprostética atrás do prato posterior e atrás do cilindro ótico, afinamento e lacunas na córnea doadora (melt) e graus de ângulo fechado.
Resultados: Os diagnósticos pré-operatórios inclui queimadura química (55%), falência pós transplante de córnea (36%) e síndrome de Stevens Johnson (9%). A idade média foi de 58.4 anos (escala, 34-83 anos). A média de tempo de pós-operatório foi de 38.5 meses (escala, 12-72 meses). Os achados mais frequentes de AS-OCT foram: membrana retroprostética, 63% (7/11); afinamento da córnea doadora (melting), 55% (6/11); angulo estreito ou fechado, 91% (10/11). Outros achados menos comuns foram crescimento epitelial sobre a superfície ótica e cistos periprostéticos. Todos os pacientes com melting (6/11) apresentaram membrana retroprostética.
Conclusões: O exame pós-operatório e a visualização detalhada das mudanças em olhos com KPro pela lâmpada de fenda pode ser difícil. AS-OCT é uma técnica de imagem útil, não invasiva e quantitativa que permite o monitoramento da estabilidade anatômica no seguimento de KPro implantadas.
Descritores: Ceratoprótese; Doenças da córnea; Próteses visuais; Tomografia de coerência óptica; Seguimento anterior do olho
INTRODUCTION
Keratoprosthesis (KPro) surgery has been increasingly accepted as a viable option for the management of patients with corneal opacification and a poor prognosis for traditional keratoplasty(1). Indications for KPro have broadened to include unilateral or bilateral corneal opacification after repeated graft failure(2), ocular trauma(3,4), herpetic keratitis(5), limbal stem cell deficiency, aniridia(6), Stevens-Johnson syndrome(7), silicone oil keratopathy, and congenital corneal opacification(8,9).
Regardless of good anatomical and functional results of KPro surgery reported by multicenter studies, major complications were also reported in a number of cases(10,11). Nonetheless, the maintenance of useful vision after KPro surgery remains challenging due to postoperative adverse events, especially those related to glaucoma, endophthalmitis/keratitis, retroprosthetic membrane formation, retinal detachment, and recurrent epithelial defects predisposing to corneal sterile necrosis and KPro extrusion(12-15). Recent studies suggest that retroprosthetic membrane formation may predispose to sterile corneal melting of the carrier corneal button, which is especially important because of the association with device extrusion(16). Routine slit lamp biomicroscopy is insufficient to fully evaluate the details of the KPro-donor corneal interface and detect retroprosthetic membrane formation.
Anterior segment optical coherence tomography (AS-OCT) allows the measurement and visualization of microscopic structures of the anterior segment, including detailed visualization of the implanted KPro and its relationship with surrounding tissues(17). The purpose of this study is to report postoperative findings of high-resolution AS-OCT of the anterior segment of eyes implanted with a type 1 Boston KPro. We emphasized on the identification of retroprosthetic membrane formation to elucidate any associations with sterile corneal melting.
METHODS
The study protocol was approved by the Institutional Review Board of the Universidade Federal de São Paulo, Brazil, and informed consent was obtained from all study participants.
Outcomes of Boston type 1 KPro implantation in a total of 11 eyes of 11 patients chosen from a population of 30 eyes were retrospectively reviewed. All procedures were performed by a single corneal surgeon (L.A.O). KPro placement was performed following a previously reported technique using a donor corneal button oversized by 0.5 mm. AS-OCT images were acquired at a single post-operative time point (Visante; Carl Zeiss Meditec AG, Jena, Germany) using the anterior segment single, double, and/or quad scan protocols by one examiner (N.A.) under standard lighting. Scans were acquired at eight representative meridians and a 360º scan of the anterior segment was obtained when possible. The presence of peripheral anterior synechiae and the number of clock hours of involvement were recorded. Postoperative AS-OCT was not routinely performed, but reserved for suspected minimal anatomical changes near the KPro-donor corneal interface (11 of 30 KPro-implanted eyes).
The main outcomes measures included retro-backplate and retro-optic membrane formation, thinning and gap formation (melting) of the corneal carrier graft, and degree of angle closure/peripheral anterior synechiae.
RESULTS
Preoperative indications for KPro surgery included chemical burn (6/11 eyes, 55%), failed corneal transplant (4/11, 36%), and Stevens-Johnson syndrome (1/11, 9%). A pseudophakic KPro was implanted in eight eyes (73%), while three eyes (27%) were implanted with aphakic devices. Ten eyes (91%) were implanted with a polymethylmethacrylate posterior back plate device (diameter, 8.5 mm) and one (9%) with a titanium back plate device (diameter, 8.5 mm). The mean ± standard deviation (SD) postoperative period to high-resolution AS-OCT was 38.5 ± 22 months (range, 12-72 months). Best-corrected visual acuity was better than 20/200 in 45% (5/11) of patients and better than 20/40 in 27% (3/11). No patient required device removal over the study duration.
Anterior segment OCT findings
Thinning and gap formation (melting) of a corneal carrier graft was observed in 6 (55%) of the 11 eyes (Figures 1 A and 2). A clinically detectable gap between the corneal carrier graft and KPro was observed by slit lamp biomicroscopy in 3/11 (28%) eyes. Suspected thinning of the graft, characterized by the presence of small air bubbles between the donor cornea and KPro edge, was observed in 1/11 (9%) eye.
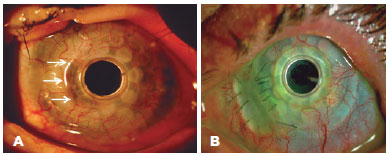
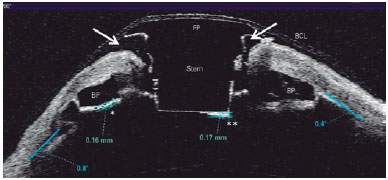
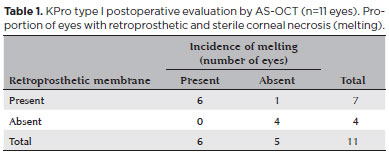
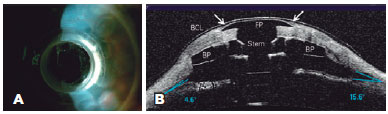
Retroprosthetic membrane formation was observed in 63% (7/11) of the eyes (Figures 1 B and 2), all of which were retro-backplate membranes, while retro-optic membrane formation was observed in 71% (5/7). Notably, all eyes with sterile corneal necrosis presented with a retroprosthetic membrane (Table 1). Most of the eyes (91%, 10/11) had some degree of peripheral anterior synechiae and angle closure (mean, 233.18º or 8.0 clock hours; range, 135-360º or 4.5-12 clock hours; SD, 118.74º) (Figure 2). Other less common findings were epithelial cyst formation and epithelial growth over the KPro optic surface (Figures 3 A and 3 B).
DISCUSSION
In agreement with previous reports in the literature, the results of the present study demonstrated good anatomical and functional results. Best-corrected visual acuity of 20/200 or better and 20/40 or better was achieved in 45% and 27% of patients, respectively. A recent literature review by the American Academy of Ophthalmology reported similar results of visual acuity of 20/200 or better after surgery in 54% to 84% of patients with a retention rate of 65% to 100% based on a follow-up duration ranging from 2 to 47 months(9). Regardless of the good anatomical and functional results, several studies have described potential vision-threatening complications, such as retroprosthetic membrane formation, elevated intraocular pressure, glaucoma progression, periprosthetic corneal melting, endophthalmitis, extrusion of the KPro, and retinal detachment after successful KPro implantation(9,11,14,18,19).
Furthermore, postoperative evaluation by slit lamp biomicroscopy has been limited to observe the interactions of keratoprostheses with the surrounding anterior segmental structures. AS-OCT offers an important imaging modality to evaluate the anterior segment anatomy after KPro implantation, which has already been well documented in previously published case series(17,20-23). AS-OCT is also used to evaluate the components of the assembled KPro in vivo by visualization of the donor-recipient corneal interface, optical cylinder, corneal graft, and angle status, as well as the presence of synechiae, the periprosthetic anatomy of KPro-implanted eyes, and early detection of important known complications(23).
In this study, retroprosthetic membrane formation was detected in 63% of the eyes by AS-OCT. In a larger series (30 eyes of 30 patients), the incidence of retroprosthetic membrane formation, as detected by slit lamp biomicroscopy, was 26.6%(24). A recent study reported retroprosthetic membrane formation, as identified by slit lamp biomicroscopy, ranging from 1% to 65%(9). Shapiro and colleagues reported the occurrence of retroprosthetic membrane in 77% of KPro eyes by AS-OCT(23,24). Moreover, AS-OCT tended to detect pre-clinical retroprosthetic membrane formation. In this series, all (100%) patients with retroprosthetic membrane formation, as detected by AS-OCT, developed retro-backplate membranes, while 71% (5/7) had developed retro-optic membranes. Another relevant findings of the present study is that all patients with sterile corneal necrosis (melting) had a detectable retroprosthetic membrane, as observed by AS-OCT. Similar results were reported by Sivaraman et al.(25), who reported AS-OCT evidence of retro-backplate membrane formation in 100% of eyes with periprosthesis melting and in 34.1% of eyes without. In agreement with this previous study, we suppose that the formation of a retro-backplate membrane in eyes implanted with a type 1 Boston KPro could be related to the development of corneal keratolysis. Thinning of the corneal carrier graft was observed in 55% of the eyes enrolled in this study, which was greater than the incidence of sterile keratolysis in our previous report of 20%, although not all patients underwent AS-OCT evaluation in that study(24). Lee and colleagues reported that the incidence of corneal melting varied from 2.4% to 30.4%(9,24).
Another finding observed in this series was that 91% of the eyes presented peripheral anterior synechiae with angle narrowing by an average of 8.0 clock hours on AS-OCT. Kang et al. reported an average of 8.7 clock hours of angle closure and 86.5% of peripheral anterior synechiae in 25 eyes implanted with a KPro and successful imaging of all meridians(17) Qian et al. demonstrated that the average degree of angle closure and peripheral anterior synechiae had increased after KPro implantation. However, these anatomic changes, as detected by AS-OCT, were not correlated to glaucoma progression(26).
A less common finding was epithelial growth over the anterior KPro surface. This finding was also reported by Kiang et al. using immunohistochemical analysis and AS-OCT, who concluded that the transparent tissue layer covering the anterior surface of the KPro front plate was found to represent non-keratinized squamous epithelium, most likely originating from the corneal epithelium(22). In a small case series by Zarei-Ghanavati et al. of the KPro-donor corneal interface by ultra-high-resolution AS-OCT suggested that the presence of gaps and lack of epithelial growth over the KPro edge might be a risk for endophthalmitis(27).
There were a few limitations to this study that should be addressed. First, the sample size was relatively small, mainly due to economic and social issues, and the study was retrospective. Second, there may have been bias regarding selection of patients to undergo AS-OCT, which was limited to patients with minimal anatomical changes, such as suspected corneal thinning, epithelial defects, and formation of air bubbles behind the anterior plate edge.
In summary, postoperative ophthalmologic examination and detailed visualization of subtle changes to the KPro-implanted eyes are often impaired by slit lamp biomicroscopy. AS-OCT is a useful noninvasive and quantitative imaging technique that allows monitoring of the anatomic stability and may help to anticipate and understand vision-threatening complications in KPro patients.
REFERENCES
1. Dohlman CH, Schneider HA, Doane MG. Prosthokeratoplasty. Am J Ophthalmol. 1974;77(5):694-700.
2. Ma JJ, Graney JM, Dohlman CH. Repeat penetrating keratoplasty versus the Boston keratoprosthesis in graft failure. Int Ophthalmol Clin. 2005;45(4):49-59.
3. Tuft SJ, Shortt AJ. Surgical rehabilitation following severe ocular burn. Eye. 2009;23(10):1966-7.
4. Harissi-Dagher M, Dohlman CH. The Boston Keratoprosthesis in severe ocular trauma. Can J Ophthalmol. 2008;43(2):165-9
5. Khan BF, Harissi-Dagher M, Pavan-Langston D, Aquavella JV, Dohlman CH. The Boston keratoprosthesis in herpetic keratitis. Arch Ophthalmol. 2007;125(6):745-9.
6. Lee H, Khan R, O'Keefe M. Aniridia: current pathology and management. Acta Ophthalmol. 2008;86(7):708-15.
7. Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston Keratoprosthesis in Stevens-Johnson syndrome, Am J Ophthalmol. 2008; 145(3):438-44.
8. Aquavella JV, Gearinger MD, Akpek EK, McCormick GJ. Pediatric keratoprosthesis. Ophthalmology. 2007;114(5):989-94.
9. Lee WB, Shtein RM, Kaufman SC, Deng SX, Rosenblatt MI. Boston keratoprosthesis: outcomes and complications. A report by the American Academy of Ophthalmology. Ophthalmology. 2015; 122(7):1504-11.
10. Rudnisky CJ, Belin MW, Guo R, Ciolino JB. Visual acuity outcomes of the Boston keratoprosthesis type 1: multicenter study results. Am J Ophthalmol. 2016;162:89-98.e1.
11. Zerbe BL, Belin MW, Ciolino JB. Results from the Multicenter Boston Type 1 Keratoprosthesis Study. Ophthalmology. 2006;113(10): 1779.e1-7.
12. Magalhães FP, Hirai FE, Sousa LB, Oliveira LA. Boston type 1 Keratoprosthesis outcomes in ocular burns. Acta Ophthalmol. 2013; 91(6):432-6.
13. Kamyar R, Weizer JS, de Paula FH, Stein JD, Moroi SE, John D, et al. Glaucoma associated with Boston type I Keratoprosthesis. Cornea. 2012;31(2):134-9.
14. Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 Keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118(8):1543-50.
15. de la Paz MF, Stoiber J, de Rezende Couto Nascimento V, Toledo JA, Seyeddain O, Hitzi W, et al. Anatomical survival and visual prognosis of Boston type 1 keratoprosthesis in challenging cases. Graefes Arch Exp Ophthalmol. 2014;252(1):83-90.
16. Harissi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea. 2007;26(5):564-8.
17. Kang JJ, Allemann N, Vajaranant TS, de la Cruz J, Cortina MS. Anterior segment optical coherence tomography for the quantitative evaluation of the anterior segment following Boston keratoprosthesis. PLoS One. 2013;8(8):e70673. doi: 10.1371/journal.pone. 0070673. Print 2013. Erratum in: PLoS One. 2013;8(9).
18. Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston Type I Keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116(4):640-51.
19. Chew HF, Ayres BD, Hammersmith KM, Rapuano CJ, Laibson PR, Myers JO et al. Boston Keratoprosthesis outcomes and complications. Cornea. 2009;28(9):989-96.
20. Garcia JP Jr., Ritterband DC, Buxton DE, de la Cruz J. Evaluation of the stability of Boston type I keratoprosthesis-donor cornea interface using anterior segment optical coherence tomography. Cornea. 2010;29(9):1031-5.
21. Fernandez AG, Radcliffe NM, Sippel KC, Rosenblatt MI, Sood P, Starr CE, et al. Boston type I keratoprosthesis-donor cornea interface evaluated by high-definition spectral-domain anterior segment optical coherence tomography. Clin Ophthalmol. 2012;6:1355-9.
22. Kiang, L, Rosenblatt MI, Sartaj R, Fernandez AG, Kiss S, Radcliffe M, et al. Surface epithelialization of the type I Boston keratoprosthesis front plate: immunohistochemical and high-definition optical coherence tomography characterization. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1195-9.
23. Shapiro BL, Cortés DE, Chin KE, Li JY, Werner JS, Redenbo E, et al. High-resolution spectral domain anterior segment optical coherence tomography in type 1 Boston keratoprosthesis. Cornea. 2013; 32(7):951-5.
24. de Oliveira LA, Pedreira Magalhães F, Hirai FE, de Sousa LB. Experience with Boston keratoprosthesis type 1 in the developing world. Can J Ophthalmol. 2014;49(4):351-7.
25. Sivaraman KR, Hou JH, Allemann N, de la Cruz J, Cortina MS. Retroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston Keratoprosthesis. Am J Ophthalmol. 2013; 155(5):814-22.
26. Qian CX, Hassanaly S, Harissi-Dagher M. Anterior segment optical coherence tomography in the long-term follow-up and detection of glaucoma in Boston type I keratoprosthesis. Ophthalmology. 2015; 122(2):317-25.
27. Zarei-Ghanavati S, Betancurt C, Mas AM, Wang J, Perez VL. Ultra high resolution optical coherence tomography in Boston type I keratoprosthesis. J Ophthalmic Vis Res. 2015;10(1):26-32.
Submitted for publication:
February 13, 2017.
Accepted for publication:
August 8, 2017.
Funding: This study was supported by Ministry of Education - Brazil (CAPES - Post Doctorate National Program 2374/2011).
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Universidade Federal de São Paulo (# 1179/07).