

Feray Koc; Mehmet Resit Isik; Nazife Sefi-Yurdakul
DOI: 10.5935/0004-2749.20180006
ABSTRACT
Purpose: To evaluate the correlation between weight reduction and visual outcome in overweight patients with idiopathic intracranial hypertension.
Methods: Thirty-nine newly diagnosed, overweight (body mass index >25 kg/m2) patients with idiopathic intracranial hypertension were studied retrospectively. All patients underwent medical treatment with acetazolamide, and a weight reduction program was also offered. Patients were grouped according to their compliance with this weight reduction program into the diet-success (Group 1) and diet-failure groups (Group 2). Body mass index, papilledema, visual acuity, and perimetric mean deviation were compared at the end of the 6-month study period.
Results: Groups 1 and 2 did not differ regarding the baseline mean body mass index (32.63 ± 5.61, 32.35 ± 5.06 kg/m2), visual acuity (0.080 ± 0.13, 0.130 ± 0.24 logMAR), perimetric mean deviation (-9.978 ± 0.68, -12.86 ± 8.91), or papilledema grade (2.94 ± 0.22, 2.90 ± 0.30), respectively (p>0.05). During the 6 months' follow-up, Group 1 patients, who complied with both medical and diet therapy, improved significantly in all parameters, including body mass index (p<0.001), visual acuity (p=0.001), perimetric mean deviation (p=0.016), and papilledema grade (p<0.001). Conversely, Group 2 patients, who only underwent medical therapy, improved only in papilledema grade (p<0.001). However, coincident development of optic disc pallor was observed in three patients. Further, they also had significant loss in visual acuity (p=0.047) during the study period.
Conclusion: Weight reduction combined with medical treatment is associated with significantly better improvement in visual acuity, visual field, and papilledema in idiopathic intracranial hypertension patients. Compliance with an efficient diet program should be encouraged in overweight patients with idiopathic intracranial hypertension.
Keywords: Obesity/complications; Intracranial hypertension; Body mass index; Weight loss; Visual acuity; Visual field
RESUMO
Objetivo: Avaliar a correlação entre a redução de peso e o resultado visual em pacientes com hipertensão intracraniana idiopática e sobrepeso.
Métodos: Trinta e nove pacientes, recém-diagnosticados com hipertensão intracraniana idiopática e sobrepeso (índice de massa corporal >25 kg/m2), foram estudados retrospectivamente. Todos os pacientes foram submetidos a tratamento médico com acetazolamida e receberam um programa para redução de peso. Os pacientes foram classificados de acordo com o cumprimento do programa de redução de peso em: sucesso da dieta (Grupo 1) e falha da dieta (Grupo 2). Os índices de massa corporal, a papiledema, a acuidade visual e desvios médios perimétricos foram comparados no final de 6 meses.
Resultados: Os grupos não apresentaram diferenças em relação às médias da linha de base de índice de massa corporal (32,63 ± 5,61/32,35 ± 5,06 kg/m2), acuidade visual (0,080 ± 0,13/0,130 ± 0,24 logMAR), desvios médios perimétricos (-9,978 ± 0,68/-12,86 ± 8,91) e níveis de papiledema (2,94 ± 0,22/2,90 ± 0,30) (p>0,05). Durante o período de acompanhamento de 6 meses, os pacientes do grupo 1, que obedeceram as terapias médicas e dietéticas, melhoraram significativamente em todos os parâmetros, incluindo o índice de massa corporal (p<0,001), a acuidade visual (p=0,001), o desvio médio perimétrico (p=0,016) e o nível de papiledema (p<0,001). Por outro lado, os pacientes do grupo 2, que receberam apenas terapia médica, apresentaram melhoras somente no nível de papiledema (p<0,001). No entanto, observou-se o desenvolvimento coincidente de palidez de disco óptico em três pacientes. Além disso, esses pacientes também apresentaram perda significativa de acuidade visual (p=0,047) durante o período de estudo.
Conclusão: A redução de peso combinada ao tratamento médico está associada à melhora significativa das acuidades visuais, dos campos visuais e de papiledema em pacientes com hipertensão intracraniana idiopática. O cumprimento de programas de dietas eficientes deve ser encorajado em pacientes obesos com hipertensão intracraniana idiopática.
Descritores: Obesidade/complicações; Hipertensão intracraniana; Índice de massa corporal; Perda de peso; Acuidade visual; Campos visuais
INTRODUCTION
It is well known that obesity, especially recent weight gain, predisposes individuals to the development and recurrence of idiopathic intracranial hypertension (IIH); however, the exact mechanism for how obesity increases intracranial pressure (ICP) has not been identified yet(1-6). In addition, the potential for weight reduction to modify obesity and treat IIH, has been confirmed(5,7-10). In this study, we aimed to evaluate the effect of weight reduction on visual outcome in an overweight or obese newly diagnosed and medically treated IIH cohort.
METHODS
This study followed the tenets of the World Medical Association's Declaration of Helsinki, and the study protocol was approved by the ethics committee of the institution. This is a retrospective, non-interventional cross-sectional study of an IIH cohort evaluated within the past 5 years. Thirty-nine patients from this group met the following enrollment criteria:
1. Diagnosis of IIH according to the modified Dandy criteria(11,12)
2. Initial body mass index (BMI) of over 25 kg/m2
3. Treatment for IIH limited to weight reduction and acetazolamide use (500-2000 mg daily) or acetazolamide only during a minimum study period of 6 months
4. Only one lumbar puncture performed, primarily as an aid in diagnosing IIH
5. Visual acuity (VA) evaluated at each visit using a Snellen chart under standard illumination
6. Reliable visual fields (VF) obtained at each visit with a Humphrey VF analyzer
7. Papilledema graded using the Frisén(13) classification (absent: grade 0, mild: grade 1, moderate: grade 2, and marked: grade 3)
8. Weight measurements obtained at the initial examination and throughout the study period
9. No use of drugs associated with high ICP
10. Referred to the dietician for weight reduction
Clinical data were estracted from the patients' medical records. BMI was used to monitor obesity. Papilledema, VA, and perimetric mean deviation (PMD) were the main outcome measures for each patient for the first 6 months after their diagnosis. Data were evaluated for each patient for the 6 months after their diagnosis. If the patient underwent a cerebrospinal fluid diversion procedure within the study period because of progressive visual loss, only the data before the surgical intervention were used. VA, PMD, and papilledema grade values of the worst eye were used in the analysis. VA scores were converted to the corresponding logMAR (logarithm of the minimum angle of resolution) equivalents for analysis. The level of obesity was determined by BMI, which was calculated as the weight (kg) divided by the square of the height (m). Overweight was defined as a BMI of >25 kg/m2 and obesity as a BMI of >30 kg/m2. Serial measurements of obesity were analyzed for change over time from the first to the sixth month.
Statistical analysis
Statistical analyses were performed with SPSS for Windows version 15 (SPSS, Inc., Chicago, IL). All data were reported as the mean ± standard deviation (SD). Baseline continuous variables of the groups were compared using the independent samples t-test after testing the equality of variances of the groups using Levene's test. Categoric variables were compared using the chi-square test. The paired samples t-test was used to compare the baseline and final values of the study parameters. Differences with a p value of less than 0.05 were considered statistically significant.
RESULTS
Thirty-nine patients who met the inclusion criteria were investigated retrospectively. All of them were overweight or obese at diagnosis. Bilateral papilledema (range: grade 0-3) was found in 37 patients. One patient had unilateral disc edema, with the other disc appearing normal, and the remaining patient had optic atrophy on one side and optic disc edema on the other side at the time of diagnosis.
Group 1 included 19 patients who complied with the diet therapy and achieved at least 6% (4.32 ± 2.69) reduction in their BMI under the guidance of a dietician. Group 2 included 20 patients who had not complied with the diet therapy; nevertheless, they showed minor BMI changes (-0.16 ± 1.46) over the study period. The baseline parameters of the two groups are shown in table 1. The groups were similar with respect to mean age and sex distribution, and the initial BMIs, VAs, PMDs, and papilledema grades of the groups were not significantly different.
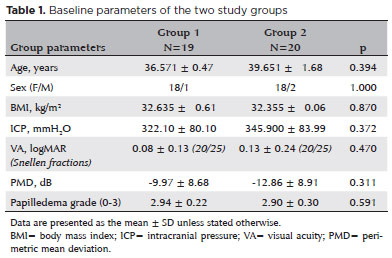
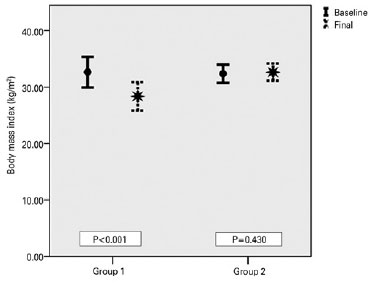
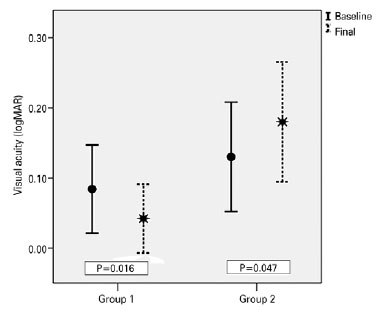
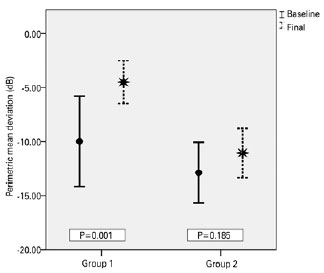
Over the follow-up period of 6 months, the patients in Group 1, who complied with both medical and diet therapy, improved significantly in all parameters (Table 2). The mean BMI decreased from 32.63 ± 5.61 to 28.34 ± 5.27 kg/m2 (p<0.001) (Figure 1), the mean VA improved from 0.084 ± 0.13 to 0.042 ± 0.10 logMAR (p=0.001) (Figure 2), and the mean PMD improved from -9.97 ± 8.68 to -4.48 ± 04.10 dB (p=0.016) (Figure 3). Conversely, the patients in Group 2, who only received medical therapy, did not improve in any of these parameters. Additionally, they showed significant VA loss (p=0.047) during the study period.
The baseline papilledema grades were 2.94 ± 0.22 and 2.90 ± 0.30 for Groups 1 and 2, respectively. Significant papilledema resolution occurred in both groups (p<0.001) by the end of the 6-month treatment period. The final papilledema grades were 0.10 ± 0.45 and 0.95 ± 1.27 for Groups 1 and 2, respectively. However, four patients in Group 2 still had grade 1-2 papilledema by the end of the study period; further, recognizable optic disc pallor was observed in the funduscopic examination of three patients in Group 2 but only in one patient in Group 1.
DISCUSSION
Progressive vision loss is the predominant morbidity of IIH, and up to 25% of IIH cases suffer from it(14). The major mechanism behind permanent optic nerve damage is axoplasmic flow stasis and the resultant intraneuronal ischemia(15,16). It is important to reverse papilledema as soon as possible, especially in cases with marked papilledema, to prevent or reverse visual loss. Currently, the initial management of papilledema in IIH includes medical therapy (e.g., acetazolamide or furosemide); however, weight loss is also recommended if the patient is overweight or obese(2,8-10,16). Newborg(9) was the first to report papilledema remission in nine patients placed on a low-calorie rice diet in 1974; however, he did not collect data on visual function. A number of subsequent studies addressed the effects of weight reduction on papilledema, ICP, visual function, and the symptoms of IIH, and significant therapeutic effects have been demonstrated on these parameters, except for visual function. Either improvement in visual function was not documented or visual function was not severely affected at the baseline examination, resulting in little improvement potential, in the groups studied(5,8-10). VA and VF deterioration usually occur over time in IIH patients. Most IIH patients do not have significant visual loss at the time of diagnosis unless they have sought medical help long after the symptoms started.
We specifically studied the effect of weight reduction on the visual function in obese IIH patients. Previously, Johnson et al.(11) reported that an approximate weight loss of 6% is associated with a 3-grade improvement in papilledema using a modified Frisén scale(13). We aimed for the same target when we grouped our patients into the diet-success group. Complete papilledema resolution was observed in 95% of the patients who had at least 6% weight reduction plus medical treatment within 6 months' follow-up. Meanwhile, only 65% of the patients who failed to adhere to the diet had complete papilledema resolution. Further, significant improvement in both VF and VA were documented in the group who complied with both diet and medical therapy. This strong correlation between weight reduction and visual function has not been demonstrated previously.Sinclair et al.(6) reported 15% weight reduction and significant improvement in ICP, papilledema, and related symptoms including headache after a low-energy diet and acetazolamide use for 3 months in 12 patients. However, there was minimal evidence of visual loss, with a mean logMAR acuity of -0.02 (± 0.10) and a mean PMD of -3.8 (± 4.1) at the start of the diet; thus, there was little potential for improvement in their patient group. Kupersmith et al.(8) also studied the association between weight reduction and grade change in papilledema and VF in 58 patients. They reported that weight reduction is associated with a more rapid recovery from both papilledema and VF dysfunction; however, the final VF outcomes did not differ between the patients who achieved at least 2.5-kg weight reduction and those who did not. Rowe and Sarkies(5) studied 34 IIH patients, and found a mean weight reduction of 3.5 kg, with only two patients losing >5% of their body weight. They did not find any association between small weight changes and visual function; however, a BMI ≥40 kg/m2 was significantly associated with poor visual outcome in their study population. The Neuro-Ophthalmology Research Disease Investigator Consortium(17) performed a multicenter study on acetazolamide therapy in 165 participants with IIH, who also received a low-sodium weight-reduction diet. They reported no significant change in VA and modest change in PMD in the participants receiving acetazolamide plus diet therapy compared with those in the diet-alone group; however, their participants also showed mild visual loss at the baseline examination compared with our group, and consequently, they had less improvement potential in their visual function. Conversely, they observed substantially greater treatment effects in participants with higher baseline papilledema grades.
Contrary to previous studies(8,17), an appreciable improvement in VF and VA could be demonstrated during treatment in the diet-success group because their baseline VA and VF were worse. On the other hand, patients in the diet-failure group did not show remarkable change in PMD; additionally, some of them continued to deteriorate in their logMAR acuity because of persistent papilledema. Although the papilledema grades improved significantly in both the diet-success and diet-failure groups, coincident development of optic disc pallor was more frequent in the diet-failure group.
Previous reports have indicated that obesity is a risk factor for longer persistence of IIH(18,19). Wong et al.(20) demonstrated an association between weight reduction and discontinuation of systemic treatment, but only after 24 months of follow-up. Our study period was not long enough to analyze the effect of obesity on the persistence of the disease; however, we could potentially discontinue medical treatment in patients who have achieved 6% weight loss, complete papilledema resolution, and stable VF.
This study has several limitations. The retrospective nature of our study might have resulted in selection bias. Although it was possible to objectively monitor the patients' compliance with the diet therapy through the recorded weight changes, we had to rely solely on the subjective statements of the patients regarding their compliance with the medical treatment. Patients who complied with diet therapy might also have been more compliant with medical treatment, whereas those who did not comply with the diet therapy might not have been compliant with the medical therapy either; this could be the reason for the worse outcomes in the diet-failure group. The use of VF testing as the main outcome measure was another study limitation. Although VF testing is the best way to follow the visual function of IIH patients, it is a subjective test and requires a high level of cooperation. Performance failure has been reported in 21% of IIH patients and in 2.7% of VF examinations overall(21). To minimize this problem, we repeated the tests if reliability indices (fixation losses, false negatives, and false positives) were not acceptable or if significant deterioration was observed compared to the previous one.
In conclusion, the results of the present study confirm that weight reduction combined with medical treatment is associated with significantly better improvement in VA, VF, and papilledema in IIH patients. Compliance with an effective diet program should be encouraged in overweight IIH patients.
REFERENCES
1. Andrews LE, Liu GT, Ko MW. Idiopathic intracranial hypertension and obesity. Horm Res Paediatr. 2014;81(4):217-25.
2. Wall M, Wall M, Kupersmith MJ, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, McDermott MP; NORDIC Idiopathic Intracranial Hypertension Study Group. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol. 2014;71(6):693-701. Comment in: JAMA Neurol. 2014;71(10):1327-8. JAMA Neurol. 2014;71(6):678-80.
3. Daniels AB, Liu GT, Volpe NJ, Galetta SL, Moster ML, Newman NJ, et al. Profiles of obesity, weight gain, and quality of life in idiopathic intracranial hypertension. Am J Ophthalmol. 2007;143(4):635-41. Comment in: Am J Ophthalmol. 2007;143(4):683-4.
4. Ireland B, Corbett JJ, Wallace RB. The search for causes of idiopathic intracranial hypertension. A preliminary case control study. Arch Neurol. 1990;47(3):315-20.
5. Rowe FJ, Sarkies NJ. The relationship between obesity and idiopathic intracranial hypertension. Int J Obes Relat Metab Disord. 1999;23(1):54-9.
6. Sinclair AJ, Burdon MA, Nightingale PG, Ball AK, Good P, Matthews TD, et al. Low energy diet and intracranial pressure in women with idiopathic intracranial hypertension: prospective cohort study. BMJ. 2010, 341:c2701. doi:10.1136/bmj.c2701.
7. Ko MW, Chang SC, Ridha MA, Ney JJ, Ali TF, Friedman DI, Mejico LJ, et al. Weight gain and recurrence in idiopathic intracranial hypertension:a case-control study. Neurology 2011;76(18):1564-7.
8. Kupersmith MJ, Gamell L, Turbin R, Peck V, Spiegel P, Wall M. Effects of weight loss on the course of idiopathic intracranial hypertension in women. Neurology. 1998;50(4):1094-8.
9. Newborg B. Pseudotumor cerebri treated by rice reduction diet. Arch Intern Med. 1974;133(5):802-7.
10. Johnson LN, Krohel GB, Madsen RW, March GA Jr. The role of weight loss and acetazolamide in the treatment of idiopathic intracranial hypertension. Ophthalmology. 1998;105(12):2313-7. Comment in: Ophthalmology. 1999;106(9):1639. Ophthalmology. 1999;106(12):2232-3.
11. Dandy WE. Intracranial pressure without brain tumor: diagnosis and treatment. Ann Surg. 1937;106(4):492-513.
12. Wall M. Idiopathic intracranial hypertension. Semin Ophthalmol. 1995;10(3):251-9.
13. Frisén L. Swelling of the optic nerve head: a staging scheme. J Neurol Neurosurg Psychiatry. 1982;45(1):13-8.
14. Corbett JJ. Savino PJ, Thompson, Kansu T, Schatz NJ, Orr LS, et al. Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss. Arch Neurol. 1982;39(8):461-74.
15. Hayreh SS. Optic disc edema in raised intracranial pressure. V. Pathogenesis. Arch Ophthalmol. 1977:95(9):1553-65.
16. Lee AG, Wall M. Papilledema: are we any nearer to a consensus on pathogenesis and treatment? Curr Neurol Neurosci Rep. 2012; 12(3):334-9.
17. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee, Wall M, McDermott MP, Kieburtz KD, Corbett JJ, Feldon SE, Friedman DI, Katz DM, Keltner JL, Schron EB, Kupersmith MJ. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014; 311(16):1641-51. Comment in: JAMA. 2014;311(16):1618-9. JAMA. 2014;312(10):1059-60.
18. Reid AC, Thomson JA. Absence of significant endocrine deficiencies in benign intracranial hypertension. J Neurol Neurosurg Psychiatry. 1981;44(8):731-4.
19. Weisberg LA. Benign intracranial hypertension. Medicine (Baltimore). 1975;54(3):197-207.
20. Wong R, Madill SA, Pandey P, Riordan-Eva P. Idiopathic intracranial hypertension: the association between weight loss and the requirement for systemic treatment. BMC Ophthalmol. 2007;7:15 doi:10.1186/1471-2415-7-15.
21. Cello KE, Keltner JL, Johnson CA, Wall M; NORDIC Idiopathic Intracranial Hypertension Study Group. Factors affecting visual field outcomes in the idiopathic intracranial hypertension treatment trial. J Neuroophthalmol. 2016;36(1):6-12.
Submitted for publication:
June 23, 2017.
Accepted for publication:
August 22, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Izmir Katip Çelebi University (# 75/2014).