

Remzi Karadag1,2; Neslihan Sevimli1; Seydi Okumus3; Isilay Ozsoy1; Huseyin Bayramlar1; Ela Durucu4; Umit Aksoy3; Christopher J. Rapuano2
DOI: 10.5935/0004-2749.20170091
ABSTRACT
Purpose: To compare the effects of 90° and 180° conjunctival rotational autograft (CRA) techniques used in primary pterygium surgery.
Methods: Forty-five patients were included in this retrospective study. Visual acuity (VA), corneal topography, and auto-refractometer measurements, as well as detailed biomicroscopic examinations, were performed preoperatively and postoperatively. During surgery, the pterygium tissue was excised then rotated 90° in Group 1 and180° in Group 2, after which it was sutured to the bare sclera. Pterygium recurrence was defined as corneal invasion ≥1 mm.
Results: Group 1 consisted of 21 patients with a mean age of 45.1 ± 11.8 years, while Group 2 comprised 24 patients with a mean age of 47.9 ± 13.8 years. The pterygia in Group 1 were graded as more advanced than those in Group 2. A similar number of recurrences were observed in Group 1 (14.3%) and in Group 2 (16.7%). There was no statistically significant difference in terms of the preoperative and postoperative VA and astigmatism values between the two groups. There was a statistically significant improvement in the postoperative VA and astigmatism values in Group 1 and in the postoperative astigmatism values in Group 2. Although postoperative redness was more common in Group 1, no statistically significant difference was found between the groups.
Conclusion:BothCRA techniques can be successful in patients for whom it is desirable to avoid a conjunctival autograft and for patients without high cosmetic expectations.
Keywords: Autografts; Conjunctiva/transplantation; Pterygium/surgery; Transplantation; autologous/methods
RESUMO
Objetivo: Comparar os efeitos das técnicas de auto-enxerto rotacional de conjuntiva (CRA) de 90° e 180°, usadas na cirurgia de pterígio primário.
Métodos: Quarenta e cinco pacientes foram incluídos neste estudo retrospectivo. Acuidade visual (AV) pré e pós-operatória, topografia da córnea, auto-refratometria e exames biomicroscópicos detalhados foram feitos. Durante a cirurgia, o tecido de pterígio foi excisado e o mesmo tecido foi girado 90° no Grupo 1 e 180° no Grupo 2, após o que foi suturado à esclera nua. A recorrência do pterígio foi definida como invasão da córnea ≥1 mm.
Resultados: O Grupo 1 consistiu em 21 pacientes, cuja média de idade foi de 45,1 ± 11,8 anos e o Grupo 2 compreendeu 24 pacientes, cuja idade média foi de 47,9 ± 13,8 anos. O Grupo 1 teve maior frequência de pterígios classificados como mais avançada do que no Grupo 2. Um número similar de recorrências foi observado no Grupo 1 (14,3%) e no Grupo 2 (16,7%). Não houve diferença estatisticamente significativa em termos de valores pré e pós-operatórios de AV e astigmatismo entre dois grupos. Houve uma melhora estatisticamente significativa nos valores pós-operatórios de AV e astigmatismo no Grupo 1 e nos valores de astigmatismo pós-operatório no Grupo 2. Embora a vermelhidão pós-operatória tenha sido detectada mais comumente no Grupo 1, não foi encontrada diferença estatisticamente significante entre os grupos.
Conclusão: Ambas as técnicas de CRA podem ser bem sucedidas em pacientes onde é desejável evitar um auto-enxerto conjuntival livre e para quem a expectativa de cosméticos não é alta.
Descritores: Autoenxertos; Pterígio/cirurgia; Conjuntiva/transplante; Transplante autólogo/métodos
INTRODUCTION
A pterygium is a triangular fibrovascular degeneration of bulbar conjunctival tissue that progresses over the limbus into the cornea(1). Although the pathogenesis of pterygia is not fully clear, possible causes include actinic degeneration triggered by ultraviolet (UV) light or subepithelial hyperplasia and basal epithelial-mesenchymal metaplasia(2). The causes, such as limbal deficiency, conjunctival-corneal epithelization(3) likely to be seen in chemical burns, virus(4), p53 tumor suppressor gene abnormality(5), and chronic inflammation(6), are also thought to play a role in the pathology of pterygium.
Indications for the surgical treatment of a pterygium include visual loss, cosmetic problems, difficulty with contact lens wear, restricted ocular motility, and chronic inflammation. Several techniques have been used in the surgical management of pterygia. Since primary excision performed using the bare sclera method is a rapid and simple technique, it has been practiced for many years. However, high rates of recurrence ranging between 29.2% and 88.9% have been reported(1,2,7). One of the methods most commonly used for preventing recurrence in clinical practice is the conjunctival autograft (CAG). CAG is an effective and reliable technique with recurrence rates between 2% and 39%(8-11). The variations of conjunctival mobilization procedures include such methods as sliding conjunctival flap(9), "narrow strip" CAG(10), limbal CAG(11), and conjunctival rotational autograft (CRA)(12). In addition to these techniques, applications of intraoperative and postoperative mitomycin-C (MMC) as well as beta radiation and amniotic membrane grafting have been used to minimize recurrence(13). Even though CAG is a reliable and effective method, it has some drawbacks. The inferior bulbar conjunctiva is not a favorable donor area because of the difficulty in performing large and thin grafts. Moreover, it has been reported in some studies that symblephara have occurred in the wake of pterygium surgery in some patients in whom autografts were taken from the inferior conjunctiva(14). Harvesting the superior bulbar conjunctiva, in contrast, is not advisable in patients who previously underwent glaucoma trabeculectomy or tube shunt surgery or who may need such surgery in the future. CAG may also not be an ideal method in eyes that require large or multiple grafts (for instance, patients with pterygia in the temporal and nasal areas)(15). In such cases, CRA is a reasonable alternative to CAG with good efficiency and low recurrence rates. In this method, which was first described by Spaeth in 1926, the pterygium tissue, after having been excised, is re-sutured to the same scleral area with 90° rotation with the head pointing upward and the base pointing downward(16).
In this study, we aimed to compare the effects of 90° and 180° CRA techniques used in primary pterygium surgery on postoperative visual acuity (VA), corneal topography, astigmatism, recurrence, and symptoms.
METHODS
Inclusion criteria and data collection: This prospective multicenter study was conducted collectively by the medical faculty at Istanbul Medeniyet University School of Medicine and Gazi Antep University. Approval was received from the ethics committee for this study. Patients over 18 years old, who had primary pterygia, were enrolled in the study. Those who had recurrent pterygium, systemic auto-immune diseases, previous limbal surgery, glaucoma, uveal or retinal diseases, degenerative or dystrophic corneal diseases likely to affect astigmatism or ocular surface disorders were excluded from the study. After informed consent was received from the patients, detailed examinations were performed.
Preoperative evaluation: The ages and genders of the patients as well as the eye (right or left) and side (nasal or temporal) in which the pterygium was located were all documented preoperatively. The preoperative Snellen VA was measured at 6 m. Auto-refractometer values were obtained using a Canon TX-20P (Tokyo, Japan). Detailed biomicroscopic anterior segment and dilated fundus examinations were performed. Corneal topographies were performed using the Sirius (Costruzione Strumenti Oftalmici, Florence, Italy) device. If a pterygium was ≤2 mm inside the cornea, it was classified as Stage 1, 2-4 mm was classified as Stage 2, and ≥4 mm was classified as Stage 3.
Surgical techniques: During the operation, the patients whose conjunctival grafts were sutured at 90° rotation were classified as Group 1, whereas those sutured at 180° rotation were classified as Group 2. Ninety-degree rotation surgeries were performed at Istanbul Medeniyet University, and 180° rotation surgeries were performed at Gazi Antep University. Subconjunctival anesthesia (epinephrine and lidocaine) was administered to all patients. Afterward, the portion of the pterygium toward the canthal side was marked with a marking pen.
The part of the pterygium extending toward the cornea was excised after separation from the cornea with a crescent blade. The fibrovascular tissue and Tenon's capsule below the conjunctival tissue were dissected and removed from the conjunctiva as much as possible, thereby disengaging the conjunctiva. The fibrovascular tissue and Tenon's capsule were also dissected from the underlying sclera, leaving a smooth and avascular bed. The disengaged conjunctiva was rotated 90° with the head pointing upward and the base pointing downward in Group 1 (180° in Group 2) and was then sutured with 7-10 interrupted 10-0-nylon sutures in its own bed onto the bare sclera (Figure 1). During the operation, no cauterization was performed. We used a similar 90° rotational technique as Speath; however, we removed the subconjunctival tissue.
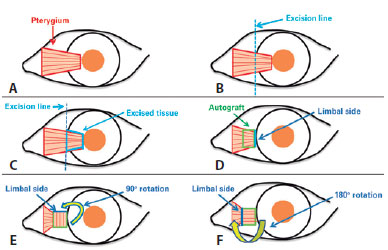
Postoperative evaluation: Postoperatively, all patients used antibiotic eye drops four times a day for 1 week and steroid eye drops in tapering doses (starting with four times a day) for 3-4 weeks. On postoperative day 1 and in the first, third, and sixth postoperative months, a follow-up visit was made. At each visit, VA and auto-refractometer measurements and biomicroscopic examinations were performed. At the sixth month visit, corneal topography was performed, and images of the anterior segment were obtained. Corneal invasion ≥1 mm was recorded as recurrence. Any injection was also documented. While a satisfactory grading system for conjunctival inflammation is not available, we established the presence or absence of injection/inflammation preoperatively and postoperatively according to the patients' perceptions and our clinical assessments (Figures 2 and 3).
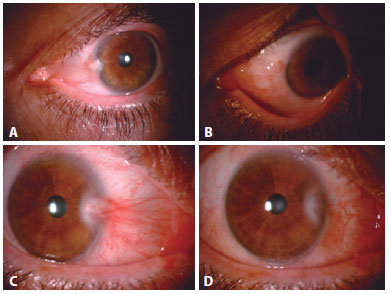
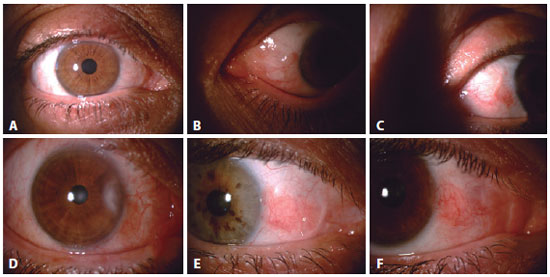
Statistical analysis: Statistical analyses were performed using SPSS software version 16 (SPSS Inc., Chicago, IL, USA). Descriptive statistical methods (mean, standard deviation, median, frequency, ratio, minimum, maximum) and parametric and nonparametric tests were used in the evaluation of this study's data. Snellen vision values were converted to logMAR for statistical analysis. Between-group comparisons were assessed for nominal variables with the Student's t test and nonnormal variables were evaluated with the Mann-Whitney U test. p<0.05 was considered statistically significant.
RESULTS
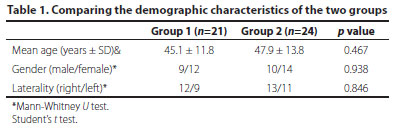
Baseline characteristics: A total of 45 patients, 21 (9 females and 12 males) in Group 1 and 24 (10 females and 14 males) in Group 2, were included in the study. The mean ages of the patients were 45.1 ± 11.8 years in Group 1 and 47.9 ± 13.8 years in Group 2. The right/left eye ratio in Group 1 was 12/9, whereas it was 13/11 in Group 2. There was no statistically significant difference between the two groups in terms of age, gender, and the nasal and temporal location where the surgery was performed (Table 1). As for the stages of pterygium: Group 1 had 1 Stage 1 eye (4.8%), 13 Stage 2 eyes (61.9%), and 7 Stage 3 eyes (33.3%). Group 2 had 11 Stage 1 eyes (45.8%), 10 Stage 2 eyes (41.7%), and 3 Stage 3 eyes (12.5%). Group 1 had statistically significantly higher stages of pterygia than Group 2 (p=0.002) (Table 2). The mean follow-up period in Group 1 was 7.8 ± 1.5 months and 6.9 ± 1.5 months in Group 2 (p=0.081).
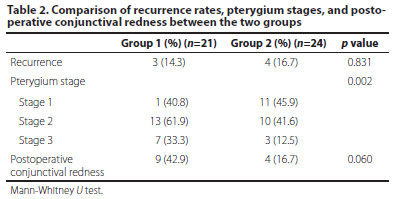
Surgical outcomes: Recurrences were observed in three patients (14.3%) in Group 1 and in four patients (16.7%) in Group 2 (p=0.831). Although postoperative redness was detected more frequently in Group 1, which was thought to be caused by the fact that the stages of pterygium in Group 1 were higher than those in Group 2 (Table 2), no statistically significant difference was found.
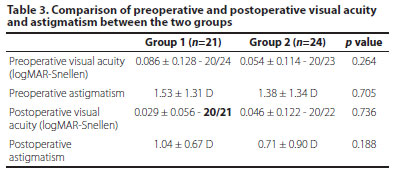
In Group 1, the best-corrected VA went from 0.086 ± 0.128 logMAR (20/24 Snellen) preoperatively to 0.029 ± 0.056 logMAR (20/21 Snellen) postoperatively. In Group 2, the value was 0.054 ± 0.114 logMAR (20/23 Snellen) preoperatively and 0.046 ± 0.122 logMAR (20/22 Snellen) postoperatively. No statistically significant difference was found between the two groups in terms of preoperative VA, postoperative VA, preoperative astigmatism, and postoperative astigmatism values (Table 3).
When the groups were compared within themselves in terms of preoperative and postoperative values, it was determined that the postoperative VA in Group 1 was statistically significantly better than the preoperative VA (p=0.005). Moreover, the postoperative astigmatism in Group 1 was found to be statistically significantly lower than the preoperative value (p<0.001). Although there was a slight improvement in the postoperative vision in Group 2, the difference was not statistically significant. The postoperative astigmatism in Group 2, however, was found to be statistically significantly lower than the preoperative value (p<0.001; Table 4).
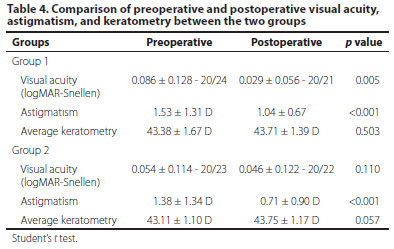
The mean preoperative K value in Group 1 was 43.38 ± 1.67 D, while the mean postoperative K value was found to be 43.71 ± 1.39 D (p=0.503). The mean preoperative K value in Group 2 was 43.11 ± 1.10 D, whereas the mean postoperative K value was found to be 43.75 ± 1.17 (p=0.057). The differences between the preoperative and postoperative average K values in Groups 1 and 2 were not statistically significant (Table 4).
DISCUSSION
CRA in pterygium surgery is one of the techniques used to minimize recurrence. This surgical method was first defined and described by Spaeth in 1926(16). Since no graft is taken from the superior bulbar conjunctiva in this method, the normal superior conjunctiva is preserved. However, since the conjunctiva was rotationally sutured without dissecting the subconjunctival fibrovascular pterygium tissue, the postoperative results of this original method are poor in terms of cosmesis. For this reason, the technique has remained unpopular(15). Jap et al. modified this technique by dissecting the subconjunctival pterygium tissue from the conjunctiva and the sclera underneath and by suturing the conjunctiva following a 180° rotation(14).
Pterygium is thought to be caused by a deficiency of limbal stem cells in the interpalpebral region exposed to chronic UV rays(3). In CAG, healthy limbal cells taken from the superior bulbar conjunctiva minimize the limbal deficiency in this region. The role of CAG in minimizing recurrence can be explained by this theory. However, since no limbal cell change occurs in CRA, other mechanisms likely play a role in the successful minimization of recurrence. According to the barrier theory defined by Youngson, trans-limbal migration takes place from the corneal epithelium toward the bare sclera in the wake of a simple excision of pterygium, which is the cause of the recurrence seen in primary pterygium surgery. Healthy limbal cells prevent the subconjunctival and conjunctival tissue from spreading over the cornea by functioning as a barrier. According to Jap et al., this barrier is damaged in patients with pterygia. The relatively normal conjunctival tissue in the canthal region enables the restoration of this barrier through physical and physiological barricades(14).
CRA is one of the treatment modalities applied to minimize pterygium recurrence. In a study conducted by Jap et al.,(14) the recurrence rate was 4%, whereas in Alp et al.'s study(17), the recurrence rate was 16.6%. In our study, however, this rate proved to be 14.3% in Group 1 and 16.7% in Group 2. The similarity of these rates to those in the study conducted by Alp et al.(17) suggests that race and environmental conditions could have an effect on recurrences. In Young et al.'s study, in contrast, the recurrence rate was lower than that in other studies. The probable reason is that intraoperative MMC was applied in addition to CRA in this study. In the study by Young et al., the fact that vascular tissue passing beyond the limbus but remaining below <1.5 mm was not defined as recurrence was regarded as a weakness of the study(2).
Graft injection is one of the major disadvantages of CRA as it may cause cosmetic issues. Although it is not difficult for ophthalmologists to detect the presence of a graft injection, there is still no satisfactory grading system concerning this finding. In our study, the graft injection rate at month 6 was found to be 42.9% in Group 1 and 16.7% in Group 2; however, the difference was not statistically significant. This difference was thought to have resulted from the fact that the number of Stage 2 and Stage 3 patients was higher in Group 1 than in Group 2. In the study conducted by Young et al., the injection rate after grafting was 96.3% at month 1, declining to 80.3% at month 6 and further declining to 61% after 1 year(2).
The fact that in Young et al.'s study, 90% of the patients stated that they saw their eyes as white or slightly injected indicate that the surgeon's perception of injection may not be the same as the subjective impression of the patient(2). The authors reported in this study that the use of intraoperative MMC did not reduce the injection rate(2). In Jap et al.'s study(14), the injection rate at month 3 was 50%, whereas in Wu et al.'s study(8), this rate was 61% at year 1, and in Dadeya et al.'s study(18), it was 5.88% at year 1. It is clear that injection rates differ dramatically in these studies. One reason for this is that there is no standard reproducible evaluation system for conjunctival injection. Pterygia may cause visual impairment by inducing regular or irregular corneal astigmatism. It is thought that the flattening effect of pterygium in the cornea occurs due to a mechanical pull or due to the pooling of tears at the apex of the pterygium(19). Budak et al. reported that there was steepening in the horizontal meridian, an increase in the central corneal curvature, and a decline in the total corneal astigmatism after pterygium surgery(20). In our study, when the mean preoperative and postoperative K values in Groups 1 and 2 were compared, the mean postoperative K values increased by a statistically insignificant amount in both groups; that is, the corneas became slightly steeper. In the study conducted by Bahar et al., in which the bare sclera technique was used, the preoperative astigmatism of 3.12 D decreased to 2.51 D postoperatively(21). In the study conducted by Yılmaz et al., in which four different techniques were compared, the residual astigmatism in the limbal CAG group was 2.06 D, whereas the residual astigmatism in the bare sclera technique performed with MMC was found to be 0.54 D(22). In our study, however, the preoperative corneal astigmatism in Group 1 was 1.53 D, whereas the postoperative corneal astigmatism decreased to 1.04 D. In Group 2, in contrast, the preoperative value was 1.38 ± 1.34 D, whereas the postoperative value decreased to 0.71 D. The wide variation in results between the residual astigmatism values seen in the above-mentioned studies is likely caused by the fact that different surgical techniques were used and by the differences in the patients' initial stages.
While performing surgery using the CRA technique, the dissection of the fibrovascular pterygium tissue from the overlying conjunctiva requires a meticulous technique. The pterygium tissue may be difficult to separate from the conjunctival folds, and the residual tissue may aggravate inflammation and then lead to injection. For this reason, the cosmetic results in CRA are less satisfactory compared with those in CAG. In CAG, the retraction effect can be minimized by fashioning the autograft to be slightly larger than the bare scleral bed; however, this is impossible in CRA since the original conjunctiva is simply replaced in a different orientation. For this reason, inflammatory retractions are often seen on the edges of the graft(13,15,18,22).
CONCLUSION
While the recurrence rates were rather low, cosmetic problems such as redness were noted with both CRA methods. In our study, more injection was seen in the 90° rotation group than in the 180° rotation group, although the difference was statistically insignificant. CRA can be considered an alternative to CAG for patients with low cosmetic expectations, for those who will probably require a healthy and strong superior conjunctival tissue in the future, and also under circumstances in which CAG cannot be performed.
REFERENCES
1. Cardillo JA, Alves MR, Ambrosio LE, Poterio MB, Jose NK. Single intraoperative application versus postoperative mitomycin C eyedrops in pterygium surgery. Ophthalmology. 1995;102:1949-52.
2. Young AL, Tam PM, Leung GY, Cheng LL, Lam PT, Lam DS. Prospective study on the safety and efficacy of combined conjunctival rotational autograft with intraoperative 0.02% mitomycin C in primary pterygium excision. Cornea. 2009;28(2):166-9.
3. Tseng SC. Concept and application of limbal stem cells. Eye (Lond). 1989;3(Pt 2):141-57.
4. Di Girolamo N. Association of human papilloma virus with pterygia and ocular-surface squamous neoplasia. Eye (Lond). 2012;26(2):202-11.
5. Tsai YY, Cheng YW, Lee H, Tsai FJ, Tseng SH, Chang KC. P53 gene mutation spectrum and the relationship between gene mutation and protein levels in pterygium. Mol Vis. 2005;11:50-5.
6. Lan W, Petznick A, Heryati S, Rifada M, Tong L. Nuclear Factor-κB: central regulator in ocular surface inflammation and diseases. Ocul Surf. 2012;10(3):137-48.
7. Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ. A randomised trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995;120(2):151-60.
8. Wu WK, Wong VW, Chi SC, Lam DS. Surgical management of double-head pterygium by using a novel technique: conjunctival rotational autograft combined with conjunctival autograft. Cornea. 2007;26(9):1056-9.
9. Kim S, Yang Y, Kim J. Primary pterygium surgery using the inferior conjunctival transposition flap. Ophthalmic Surg Lasers. 1998;29(7):608-11.
10. Dupps WJ Jr, Jeng BH, Meisler DM. Narrow-strip conjunctival autograft for treatment of pterygium. Ophthalmology. 2007;114(2):227-31.
11. Rao SK, Lekha T, Mukesh BN, Sitalakshmi G, Padmanabhan P. Conjunctival-limbal autografts for primary and recurrent pterygia: technique and results. Indian J Ophthalmol. 1998;46(4):203-9.
12. Sharma AK, Kumar VB. Rotational grafting of pterygium. Indian J Ophthalmol. 1984; 32(3):149-51.
13. Kim SH, Oh JH, Do JR, Chuck RS, Park CY. A comparison of anchored conjunctival rotation flap and conjunctival autograft techniques in pterygium surgery. Cornea. 2013;32(12):1578-81.
14. Jap A, Chan C, Lim L, Tan DT. Conjunctival rotation autograft for pterygium. An alternative to conjunctival autografting. Ophthalmology. 1999;106(1):67-71.
15. Sune MP, Sune PG. Conjunctival rotation autograft for pterygium: an alternative to conventional conjunctival autografting. Asia Pac J Ophthalmol (Phila). 2013;2(4):227-31.
16. Spaeth ED. Rotated island graft operation for pterygium. Am J Ophthalmol. 1926;9(9): 649-55.
17. Alp BN, Yanyali A, Ay GM, Keskin O. Conjunctival rotation autograft for primary pterygium. Ophthalmologica. 2002;216(5):333-6.
18. Dadeya S, Malik KP, Gullian BP. Pterygium surgery: conjunctival rotation autograft versus conjunctival autograft. Ophthalmic Surg Lasers. 2002;33(4):269-74.
19. Hansen A, Norn M. Astigmatism and surface phenomena in pterygium. Acta Ophthalmol (Copenh). 1980;58(2):174-81.
20. Budak K, Khater TT, Friedman NJ, Koch DD. Corneal topographic changes induced by excision of perilimbal lesions. Ophthalmic Surg Lasers. 1999;30(6):458-64.
21. Bahar I, Loya N, Weinberger D, Avisar R. Effect of pterygium surgery on corneal topography: a prospective study. Cornea. 2004;23(2):113-7.
22. Yilmaz S, Yuksel T, Maden A. Corneal topographic changes after four types of pterygium surgery. J Refract Surg. 2008;24(2):160-5.
Submitted for publication:
March 21, 2017.
Accepted for publication:
June 24, 2017.
Funding: No specific financial support was available for this study.
Disclosure of potential conflicts of interest: None of the authors have any potential conflicts of interest to disclose.
Approved by the following research ethics committee: Istanbul Medeniyet University (#2015/0060).