

Cristina Castro-Lima-Vargens1,2; Maria Fernanda Rios Grassi1,3; Ney Boa-Sorte1; Regina Helena Rathsam-Pinheiro2; Paula Caroline Matos Almeida2; Bernardo Galvão-Castro1,3
DOI: 10.5935/0004-2749.20170090
ABSTRACT
Purpose: To evaluate the accuracy of lacrimal film tests and propose an algorithm for the diagnosis of dry eye disease in individuals infected with human T-cell lymphotropic virus type 1.
Methods: Ninety-six patients infected with human T-cell lymphotropic virus type 1 were enrolled in the study. To assess clinical complaints, patients completed the Ocular Surface Disease Index questionnaire. To evaluate lacrimal film quality, patients underwent the tear breakup time test, Schirmer I test, and Rose Bengal staining. Dry eye disease was diagnosed when at least two of the three test results were abnormal. The sensitivity, specificity, positive and negative predictive values, and overall accuracy of the questionnaire as well as of each test alone and combined in parallel and in series were determined.
Results: The most sensitive test was the tear breakup time test (98%), whereas the most specific was the Schirmer I test (100%). Rose Bengal staining had the highest overall accuracy (88.64%), whereas the Ocular Surface Disease Index had the lowest overall accuracy (62.65%). The tear breakup time test, Schirmer I test, and Ocular Surface Disease Index combined in parallel showed increased sensitivity and decreased specificity for all tests. In contrast, when combined in series, these tests demonstrated increased specificity and decreased sensitivity.
Conclusion:This study shows the need to use multiple tests to evaluate tear film quality and include a symptom questionnaire as part of the diagnostic algorithm for dry eye disease.
Keywords: Keratoconjunctivitis sicca; Human T-cell lymphotropic virus 1; Dry eye syndromes/diagnosis
RESUMO
Objetivo: Avaliar a precisão da propedêutica do filme lacrimal e propor um algoritmo para o diagnóstico da doença do olho seco em indivíduos infectados com Vírus linfotrópico de células-T humanas tipo 1.
Métodos: Noventa e seis pacientes infectados com o vírus linfotrópico de células T humana tipo 1 foram incluídos no estudo. Para avaliar sintomatologia, os pacientes responderam o questionário Índice para Doenças da Superfície Ocular. A fim de avaliar a qualidade do filme lacrimal, os pacientes foram submetidos ao teste de ruptura do filme lacrimal, teste de Schirmer I e coloração com Rosa Bengala. A doença do olho seco foi diagnosticada quando, pelo menos, dois dos testes ruptura do filme lacrimal, teste de Schirmer I e coloração com Rosa Bengala) eram anormais. Foram determinados sensibilidade, especificidade, valor preditivo positivo e negativo e acurácia do questionário e de cada teste sozinho e combinados em paralelo e em série.
Resultados: O teste de ruptura do filme lacrimal foi o mais sensível (98%) e o teste de Schirmer I foi o mais específico (100%). A maior acurácia foi encontrada no teste de coloração com Rosa Bengala (88,64%), enquanto sintomas avaliados usando o questionário Índice para Doenças da Superfície Ocular teve a menor acurácia geral (62,65%). O teste de ruptura do filme lacrimal, teste de Schirmer I e Questionário de Doença da Superfície Ocular quando combinados em paralelo mostraram um aumento da sensibilidade e uma diminuição na especificidade de todos os testes. Por outro lado, combinados em série, teste de ruptura do filme lacrimal, Schirmer I e questionário Índice para Doenças da Superfície Ocular tiveram um aumento na especificidade e sensibilidade diminuída.
Conclusão: Este estudo apontou a necessidade de utilizar mais do que um teste para avaliar a qualidade do filme lacrimal, bem como utilizar um questionário de sintomas como parte do algoritmo de diagnóstico para doença do olho seco.
Descritores: Ceratoconjuntivite seca; Vírus 1 linfotrópico T humano; Síndromes do olho seco/diagnóstico
INTRODUCTION
Human T-cell lymphotropic virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia(1), HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP)(2), and infective dermatitis in children(3). It is estimated that 5-10 million people worldwide are infected with HTLV-1(4). HTLV-1-associated uveitis (HAU), an ophthalmologic disease, is also linked to HTLV-1 infection(5,6). In Japan, HAU has a prevalence of 9.5%-44.8%(7,8). Other ophthalmologic alterations such as corneal lesions, retinal vasculitis, and keratoconjunctivitis sicca (KCS) or dry eye disease (DED) are also associated with HTLV-1(9-12). In individuals with HTLV-1, the prevalence of DED may reach 30%-40%(13-16), especially in symptomatic patients with HAM/TSP.
DED is an ocular surface disease that causes eye discomfort, visual disturbance, and tear film instability(17). Aging, medications, eyelid problems, and environmental factors may be associated with DED. Diseases such as Sjögren's syndrome and rheumatoid arthritis can also cause DED(18). In these autoimmune diseases, the major finding is the destruction of lacrimal gland ducts by autoantibodies(19).
The mechanism leading to DED in HTLV-1-infected persons remains unclear. It is reported that antinuclear antibodies, such as rheumatoid factor, anti-SSA/Ro, and anti-SSB/La, which are present in autoimmune diseases, are absent in patients with HTLV-1-associated DED(20). Conversely, there is an association between a high HTLV-1 proviral load and DED in infected patients(20,21).
Complaints suggestive of DED are blurred vision, dryness, foreign body sensation, and burning eyes. To confirm the diagnosis, it is mandatory to measure tear volume and evaluate tear quality(17). Three widely used tests for the assessment of DED are the Schirmer I test, tear breakup time test (TBUT), and Rose Bengal staining(22). First described in 1903, the Schirmer I test is still being used to measure basal and reflex tear secretions(23). TBUT is used to assess tear film stability(17). Rose Bengal staining evaluates the conjunctiva and cornea for the absence of membrane-associated mucins(24). However, this test has the disadvantage of being toxic, and it typically causes a burning sensation(25). Patient complaints can also be evaluated using the Ocular Surface Disease Index (OSDI)(26), a specific questionnaire that rapidly assesses dry eye symptoms and their impact on vision-related functioning(27).
Studies to date have generally combined symptoms questionnaires and two or three tests to evaluate tear volume and quality. However, no definite protocol for DED diagnosis has been proposed, and there is a poor relationship between symptoms and diagnostic tests(22,27). The aim of the present study was to evaluate the accuracy of the TBUT, Schirmer I test, Rose Bengal staining, and OSDI alone or combined for diagnosing DED in HTLV-1-infected individuals and to propose an algorithm for diagnosing DED using low-cost and minimally invasive procedures.
METHODS
This prospective study was conducted at the Bahiana School of Medicine and Public Health reference center for HTLV, Salvador, Bahia, Brazil, between February and November 2013. Patients were sequentially invited to the ophthalmology clinic during routine medical visits. Individuals were eligible to participate if they had a positive serological diagnosis of HTLV-1 on enzyme-linked immunosorbent assay and western blotting. Patients presenting with any previous palpebral and conjunctival disorders, history of ocular surgery, active eye infection, nasolacrimal duct obstruction, contact lens use, chemical or thermal ocular burn, or pregnancy were excluded. The Institutional Research Board of Bahiana School of Medicine approved the study and all patients signed an informed consent form.
To calculate the sample size, we considered the KCS prevalence in patients with HTLV-1 to be equal to 35.0%(13-16) and confidence limits as 10.0%. Considering an alpha error of 5%, the estimated minimum sample obtained was 83 individuals.
Ophthalmologic examination and measurements
Patients underwent a detailed ophthalmic examination, including best-corrected visual acuity and intraocular pressure measurement with an applanation tonometer. The presence of uveitis was evaluated by an anterior segment and fundus examination with a slit-lamp biomicroscope.
All patients were evaluated for clinical symptoms using the OSDI. This questionnaire has been validated in Brazil(28) and provides a rapid assessment of the symptoms of ocular irritation consistent with DED. Patients were classified on a dry eye intensity scale as normal, mild, moderate, or severe according to the OSDI score. The test was considered positive if the patient's final OSDI score was >20 (moderate or severe).
Tear secretions for both eyes were evaluated using the TBUT, Schirmer I test, and Rose Bengal staining. TBUT was performed by the instillation of a 1% fluorescein solution (Colírio de Fluoresceína®; Ophthalmos®, São Paulo, Brazil), and the time required for dry spots to appear on the corneal surface after blinking was recorded. Dry spots that appeared in <10 s were considered abnormal.
To perform the Schirmer I test, a Whatman filter paper strip (Teste de Schirmer®; Ophthalmos®) with a dimension of 5 × 35 mm was placed into the lower fornix near the lateral canthus of each eye as the anesthetic was administered. After 5 min, the strips were removed and the wet portion was measured. The result was considered abnormal if <5 mm of moisture was present on the filter paper.
The Rose Bengal test was performed with 1% Rose Bengal staining solution (Colírio de Rosa Bengala®; Ophthalmos®). The nasal conjunctiva, cornea, and temporal conjunctiva were evaluated and each was scored 0-3 points. The exam was considered abnormal when the total score was >3 points(29).
DED was diagnosed when the results of at least two of the three tests were abnormal.
Statistical analysis
Age is expressed as mean (SD), while sex and the presence of DED are expressed as relative frequency. The Kolmogorov-Smirnov test was used to assess the presence of a normal age distribution. The means were compared using t-tests or the Mann-Whitney tests according to Gaussian or non-Gaussian statistical distribution. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy (OA) of each test alone were calculated using the OpenEpi software program version 3.01. OA was calculated as the sum of the true positives plus true negatives divided by the total number of individuals tested. Sensitivity was obtained by the ratio of the number of true positive assessments and the number of all positive assessments. Specificity was obtained by the ratio of the number of true negative assessments and the number of all negative assessments. PPV was defined as the proportion of patients with positive test results who were correctly diagnosed, while NPV was defined as the proportion of patients with negative test results who were correctly diagnosed. Two tests at a time were combined in parallel and in series to evaluate their ability to differentiate persons with DED from normal individuals. When combined in parallel, only one positive result was sufficient, while when combined in series, both tests must be positive. Global algorithm accuracy, sensitivity, specificity, PPV, and NPV were determined. The MS Excel software program was used to calculate accuracy. All statistical analyses were performed using SPSS/PC Statistical Software Program version 18.0 (SPSS, Chicago, IL, USA).
RESULTS
Ninety-six subjects were included in the study; of them, 71 (74%) were females. Fifty patients (52.1%) had a diagnosis of DED. The mean age for patients with the DED diagnosis was 53.6 years, while that for patients without DED was 47.4 years; the difference was statistically significant (p=0.017). The age for all patients was 23-78 years.
The sensitivity, specificity, PPV, NPV, and OA of each test alone and combined in parallel or in series are presented in table 1. When evaluated alone, the most sensitive test was TBUT (98%), presenting a false-negative rate of 3.0% and specificity of 69.6%. The Schirmer I test was the most specific (100%), with no false-positives cases and a sensitivity of 44%. Rose Bengal staining had the highest OA for sensitivity and specificity (88.6%), while OSDI had the lowest OA (62.7%).
Combined in parallel, the TBUT and OSDI were the most sensitive (99.5%), followed by the combination of the TBUT and Schirmer I test (98.9% sensitivity), with false-positive rates of 37.3% and 22.1%, respectively. The specificities of both combinations were 35.6% and 69.6%, respectively. The combination of the TBUT, Schirmer I test, and OSDI showed the highest sensitivity (35.6%).
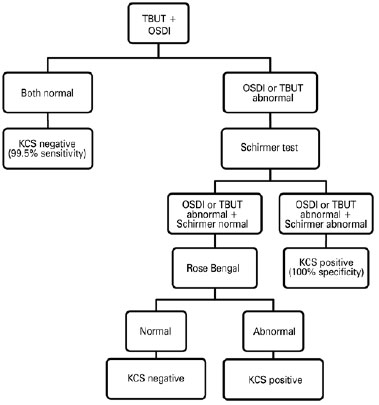
Combined in series, a positive Schirmer I test combined with a positive TBUT or OSDI test reached a specificity of 100%, with 43.1% and 33.0% sensitivity, respectively. The TBUT, Schirmer I test, and OSDI combined showed 100% specificity and 37.8% sensitivity. Global algorithm accuracy, sensitivity, specificity, PPV, and NPV are shown in figure 1. Four patients did not complete all of the ophthalmologic evaluations. For the 92 individuals tested, the algorithm's OA, sensitivity, and specificity were 95.7% (95% confidence interval [CI], 89.4-98.3), 98.0% (95% CI, 89.5-99.7), and 92.9% (95% CI, 81.0-97.5), respectively. PPV was 94.2% (95% CI, 84.4-98.0) and NPV was 97.5% (95% CI, 87.1-99.6).
DISCUSSION
The results presented herein refer to the assessment of the efficacy of low-cost tests that are widely used to diagnose DED applied to a group of HTLV-1-infected patients. Although DED is more prevalent in HAM/TSP patients(14,15), the patients in this series were subjected to the same protocol as asymptomatic patients.
When used alone, the TBUT was the best screening test, featuring the highest sensitivity and NPV. Similar results were found in patients with a diagnosis of Sjögren's syndrome but not HTLV-1(30). However, to confirm the diagnosis, additional tests were required. In the present study, the Schirmer I test showed a low sensitivity (<50%) but had the highest specificity (100%) similar to rates described in the literature(30,31). Rose Bengal staining presented the best OA. However, when evaluated alone, Rose Bengal did not provide a high PPV or NPV. Therefore, the use of this test alone is unsuitable for confirming or excluding the diagnosis of DED. Although Rose Bengal staining seems to be an efficient test for making the ophthalmic differential diagnosis of Sjögren's syndrome in KCS patients(32), it is best used as an adjunct due to its lack of sensitivity and specificity(33). Moreover, patients usually complained of itching and redness or even severe ocular inflammation after application of the Rose Bengal stain. Despite the stain being a derivative of fluorescein, which is harmless, it has a dose-dependent toxic effect on human corneal epithelial cells in vitro(24). Due to its side effects, the use of Rose Bengal stain should be limited to patients for whom the DED diagnosis is inconclusive. The Rose Bengal stain can also be replaced with lissamine green, a stain that is well tolerated and as effective as Rose Bengal for evaluating the ocular surface(34).
To confirm the diagnosis of DED, symptoms of discomfort and visual disturbance should be evaluated(35). Regarding the effectiveness of the OSDI, the accuracy was very low when the questionnaire alone was used to evaluate for DED. This demonstrated a weak correlation between the patient's symptoms and clinical signs(36).
Serial testing maximizes specificity and the PPV but decreases sensitivity and the NPV. Multiple tests combined in parallel increase the sensitivity and, therefore, the NPV. Instead of performing all tests for all patients, the diagnostic algorithm proposed in the present study follows a sequence that involves performing the tests in three stages. Based on the results obtained herein, we suggest an algorithm for screening HTLV-1-infected patients and making a reliable diagnosis of KCS (Figure 1). First, all infected patients would be screened using the OSDI questionnaire and the TBUT. If both tests are normal, a diagnosis of DED can be excluded. These results corroborate those of recent studies indicating that OSDI and TBUT are important tests for diagnosing DED(35,37).
Second, if the OSDI questionnaire and/or TBUT results are abnormal, the Schirmer I test must be performed for all patients. A positive Schirmer I test confirms the KCS diagnosis. In distinct well-defined conditions, the combination use of the OSDI, TBUT, and Schirmer test was the best combination to detect DED(30).
Third, in those patients for whom the diagnosis remains indeterminate (Schirmer I test negative), Rose Bengal staining must be performed. The Japanese Diagnostic Criteria for Dry Eye considers a simultaneous positive test for OSDI, TBUT, and Rose Bengal as a definite DED diagnosis(38).
Although a 100% PPV was found for several test combinations evaluated in this study, none of the tests was sufficiently effective to completely exclude the occurrence of false negatives. Therefore, in patients with a HAM/TSP diagnosis, with an HTLV-1 proviral load of >10% of infected cells, or those >45 years of age, Rose Bengal staining should be considered, even when TBUT, OSDI, and Schirmer test results are negative.
One limitation of the present study is that the proposed algorithm cannot further distinguish DED as aqueous deficient dry eye (ADDE) or evaporative dry eye (EDE). However, it is probable that DED in patients infected with HTLV-1 is mainly due to viral damage to the lacrimal gland, resulting in decreased tear production(39,40). In addition, Meibomian gland disease, the most common form of EDE, can be easily excluded by examination of the eye structures using a biomicroscope.
In summary, this study demonstrated that a reliable DED diagnosis can be made in HTLV-1 patients using tests to evaluate DED along with a validated questionnaire, not only to obtain information about the patients' complaints but also as part of the diagnostic algorithm. The described algorithm might be useful for diagnosing moderate to severe ADDE. This would enable the exclusion of patients in whom it is possible to confirm or exclude the DED diagnosis before the next step. Therefore, this algorithm can contribute to reducing cost, discomfort, and time without compromising diagnostic efficacy.
REFERENCES
1. Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cells lines of human adult T-cell leukemia and its implications in the disease. Proc Natl Acad Sci USA. 1982;79(6):2031-5.
2. Gessain A, Barin F, Vernant JC, Gout O, Maurs L, Calender A, et al. Antibodies to human T-lymphotropic virus type I in patients with tropical spastic paraparesis. Lancet. 1985; 2(8452):407-10.
3. Blattner W, Hanchard B, Fletcher V, LaGrenade L, Cranston B. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990;336(8727):1345-7.
4. Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388.
5. Mochizuki M, Watanabe T, Yamaguchi K, Yoshimura K, Nakashima S, Shirao M. Uveitis associated with human T-cell lymphotropic virus type I. Am J Ophthalmol. 1992;114(2): 123-9.
6. Nakao K, Ohba N, Matsumoto M. Noninfectious anterior uveitis in patients infected with human T lymphotropic virus type I. Jpn J Ophthalmol. 1989;33(4):472-81.
7. Goto K, Saeki K, Kurita M, O. Shigeaki. HTLV-I seroprevalence in patients with undefined uveitis in central Japan. Jpn J Ophthalmol. 1994;38(2):175-7.
8. Mochizuki M, Watanabe T, Yamaguchi K, Takatsuki K, Yoshimura K, Shirao M, et al. HTLV-1 uveitis: a distinct clinical entity caused by HTLV-1. Jpn J Cancer Res. 1992;83(3): 236-9.
9. Merle H, Cabre P, Olindo S, Merle S, Smadja D. Ocular lesions in 200 patients infected by the t-cell lymphotropic virus type 1 in Martinique (French West Indies). Am J Ophthalmol. 2002;134(2):190-5.
10. Merle H, Smadja D, Le Hoang P, Bera O, Cabre P, Landau M, et al. Ocular manifestations in patients with HTLV-I associated infection: a clinical study of 93 cases. Jpn J Ophthalmol. 1996;40(2):260-70.
11. Buggage RR, Levy-Clarke GA, Smith JA. New corneal findings in human T-cell lymphotropic virus type 1 infection. Am J Ophthalmol. 2001;131(3):309-13.
12. Levy-Clarke G, Buggage R, Shen DF, Vaughn LO, Chan CC, Davis JL. Human T-cell lymphotropic virus type-I associated T-cell leukemia-lymphoma masquerading as necrotizing retinal vasculitis. Ophthalmology. 2002;109(9):1717-22.
13. Nasu M, Matsubara O, Yamamoto H. Post-mortem prevalence of lymphocytic infiltration of the lacrimal gland: a comparative study in autoimmune and non-autoimmune diseases. J Pathol. 1984;143(1):5-11.
14. Pinheiro SR, Martins-Filho OA, Ribas JG, Catalan-Soares BC, Proietti FA, Namen-Lopes S, et al. Immunologic markers, uveitis, and keratoconjuntivitis sicca associated with human T-cell lymphotropic virus type 1. Am J Ophthalmol. 2006;142(5):811-5.
15. Rathsam-Pinheiro RH, Boa-Sorte N, Castro-Lima-Vargens C, Pinheiro CA, Castro-lima H, Galvão Castro B. Ocular lesions in HTLV-1infected patients from Salvador, State of Bahia: the city with the highest prevalence of this infection in Brazil. Rev Soc Bras Med Trop. 2009;42(6):633-7.
16. Merle H, Cabre P, Olindo S, Merle S, Smadja D. Ocular lesions in 200 patients infected by the human T-cell lymphotropic virus type 1 in martinique (French West Indies). Am J Ophthalmol. 2002,134(2):190-5.
17. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5(2):75-92.
18. Lemp MA. Dry eye (keratoconjunctivitis sicca), rheumatoid arthritis, and Sjögren's syndrome. Am J Ophthalmol. 2005;140(5):898-9.
19. Toshiharu H. Dysfunction of lacrimal and salivary glands in Sjögren's syndrome: nonimmunologic injury in preinflammatory phase and mouse model. J Biomed Biotechnol. 2011;2011:407031. doi: 10.1155/2011/407031.
20. Ferraz-Chaoui AK, Atta AM, Atta ML, Galvão-Castro B, Santiago MB. Study of autoantibodies in patients with keratoconjunctivitis sicca infected by the human T cell lymphotropic virus type 1. Rheumatol Int. 2010;30(6):775-8.
21. Castro-Lima-Vargens C, Grassi MFR, Boa-Sorte N, Rathsam-Pinheiro RH, Olavarria VN, Kruschewsky RA, et al. Keratoconjunctivitis sicca of human T cell lymphotropic virus type 1 (HTLV-1) infected individuals is associated with high levels of HTLV-1 proviral load. J Clin Virol. 2011;52(3):177-80.
22. Smith J, Nichols KK, Baldwin EK. Current patterns in the use of diagnostic tests im dry eye evaluation. Cornea. 2008;27(6):656-62.
23. Schirmer O. Studien zur physiologie und pathologie der tranen-absonderung und tranenabfuhr. Graefes Arch Clin Exp Ophthalmol. 1903;56:197-291.
24. Feenstra RP, Tseng SC. What is actually stained by rose bengal? Arch Ophthalmol. 1992; 110(7):984-93.
25. Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E. The challenge of dry eye diagnosis. Clin Ophthalmol. 2008;2(1):31-55.
26. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615-21.
27. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762-70.
28. Prigol AM, Tenório MB, Matsschinske R, Gehlen ML, Skare T. Translation and validation of ocular surface disease index to Portuguese. Arq Bras Oftalmol. 2012;75(1):24-8.
29. van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969; 82(1):10-4.
30. Alves M, Reinach PS, Paula JS, Vellasco e Cruz AA, Bachette L, Faustino J, et al. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. Plos One. 2014;9(5):e97921.
31. Versura P, Frigato M, Cellini M, Mulè R, Malavolta N, Campos EC. Diagnostic performance of tear function tests in Sjogren's syndrome patients. Eye (Lond). 2007;21(2): 229-37.
32. Knezovic I, Alajbeg I, Karlovic D, Pavan J, Vrkic N, Biscan A. Differential diagnostic performance of Rose Bengal score test in Sjogren syndrome patients. Coll Antropol. 2011;35(4):1105-13.
33. Schein OD, Tielsch JM, Munoz B, Bandeen-Roche K, West S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology. 1997;104(9):1395-401.
34. Manning FJ, Wehrly SR, Foulks GN. Patient tolerance and ocular surface staining characteristics of lissamine green versus rose bengal. Ophthalmology. 1995;102(12): 1953-7.
35. Tsubota K, Yokoi N, Shimazaki J, Watanabe H et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65-76.
36. McGinnigle S, Naroo AS, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012; 57(4):293-316.
37. Baudouin C, Aragona P, Van Setten G, Rolando M, Irkeç M, Benítez del Castillo J, et al. Diagnosing the severity of dry eye: a clear and practical algorithm. Br J Ophthalmol. 2014;98(9):1168-76.
38. Schimazaki J. Dry Eye Research Group. [Definition and diagnosis of dry eye 2006]. Atarashii Ganka. 2007;24:181-4. Japanese
39. Mochizuki M. Regional immunity of the eye. Acta Ophthalmol. 2010; 88(3):292-9.
40. Alves M, Angerami RN, Rocha EM. Dry eye disease caused by viral infection: review. Arq Bras Oftalmol. 2013;76(2):129-32.
Submitted for publication:
February 9, 2017.
Accepted for publication:
July 17, 2017.
Funding: This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and the Fundação de Amparo à Pesquisa do Estado da Bahia (Fapesb).
Disclosure of potential conflicts of interest: None of the authors have any potential conflict of interest to disclose.
Approved by the following research ethics committee: Escola Bahiana de Medicina e Saúde Pública (# 329/2011).