

Renata Tiemi Kashiwabuchi1; Yasin Khan2; Fabio Ramos de Souza Carvalho1; Flavio Hirai1; Mauro Silveira Campos1; Peter John McDonnell2
DOI: 10.1590/S0004-27492012000600011
ABSTRACT
PURPOSE: To assess S. aureus in vitro viability after the exposure to ultraviolet light A (UVA) and riboflavin (B2). METHODS: Samples of S. aureus in 96 well plates (in triplicate) were exposed to riboflavin (B2) and ultraviolet light A (365 nm wavelength) at a power density of 3 mW/cm², 8 mm spot diameter, for 30 minutes. Control groups were prepared as well in triplicate: blank control, ultraviolet light A only, riboflavin only and dead bacteria Control. The bacterial viability was measured using fluorescent microscopy. In order to investigate the occurrence of "viable but non-culturable" microorganisms after treatment, the cell viability was also investigated by plate culture procedure onto a broth medium. Statistical analysis was performed using the triplicate values from each experimental condition. RESULTS: No difference was observed among the treatment group and the control samples (p=1). CONCLUSION: The combination of riboflavin 0.1% and ultraviolet light A at 365 nm did not exhibit antimicrobial activity against oxacillin susceptible S. aureus.
Keywords: Keratitis; Riboflavin; Corneal ulcer; Staphylococcus aureus; Ultraviolet rays; Cross-linking reagents
RESUMO
OBJETIVO: Avaliar a viabilidade celular de S. aureus in vitro após a exposição de riboflavina (B2) e luz ultravioleta A (UVA). MÉTODOS: Amostras de S. aureus colocadas em uma placa de 96 poços (em triplicata) foram expostas a riboflavina 0,1% (B2) e luz ultravioleta (comprimento de onda de 365 nm) poder de 3 mW/cm², 8 mm de diâmetro, por 30 minutos. Grupos controles foram também preparados em triplicata: controle branco, somente luz ultravioleta A, somente riboflavina e controle morto. A viabilidade bacteriana foi analisada usando microscópio de fluorescência. Para investigar a ocorrência de micro-organismos "viáveis porem não cultiváveis" a viabilidade celular foi avaliada utilizando-se placas de meio de cultivo bacteriano. Analise estatística foi realizada utilizando-se os valores obtidos em triplicata de cada grupo experimental. RESULTADOS: Nenhuma diferença foi observada entre o grupo tratamento e os grupos controle (p=1). CONCLUSÃO: A combinação riboflavina 0,1% e luz ultravioleta 365 nm de comprimento de onda não demonstrou atividade antimicrobiana contra S. aureus oxacilina sensível.
Descritores: Ceratite; Riboflavin; Úlcera da córnea; Staphylococcus aureus; Raios ultravioleta; Reagentes para ligações cruzadas
ORIGINAL ARTICLE ARTIGO ORIGINAL
Antimicrobial susceptibility of photodynamic therapy (UVA/riboflavin) against Staphylococcus aureus
Suscetibilidade antimicrobiana da terapia fotodinâmica (UVA/riboflavina) contra Staphylococcus aureus
Renata Tiemi KashiwabuchiI; Yasin KhanII; Fabio Ramos de Souza CarvalhoI; Flavio HiraiI; Mauro Silveira CamposI; Peter John McDonnellII
IDepartment of Ophthalmology, Escola Paulista de Medicina - Universidade Federal de São Paulo - UNIFESP, São Paulo, Brazil
IIThe Wilmer Ophthalmological Institute, The Johns Hopkins University, School of Medicine, Baltimore, Maryland, USA
ABSTRACT
PURPOSE: To assess S. aureus in vitro viability after the exposure to ultraviolet light A (UVA) and riboflavin (B2).
METHODS: Samples of S. aureus in 96 well plates (in triplicate) were exposed to riboflavin (B2) and ultraviolet light A (365 nm wavelength) at a power density of 3 mW/cm2, 8 mm spot diameter, for 30 minutes. Control groups were prepared as well in triplicate: blank control, ultraviolet light A only, riboflavin only and dead bacteria Control. The bacterial viability was measured using fluorescent microscopy. In order to investigate the occurrence of "viable but non-culturable" microorganisms after treatment, the cell viability was also investigated by plate culture procedure onto a broth medium. Statistical analysis was performed using the triplicate values from each experimental condition.
RESULTS: No difference was observed among the treatment group and the control samples (p=1).
CONCLUSION: The combination of riboflavin 0.1% and ultraviolet light A at 365 nm did not exhibit antimicrobial activity against oxacillin susceptible S. aureus.
Keywords: Keratitis; Riboflavin; Corneal ulcer; Staphylococcus aureus; Ultraviolet rays; Cross-linking reagents
RESUMO
OBJETIVO: Avaliar a viabilidade celular de S. aureus in vitro após a exposição de riboflavina (B2) e luz ultravioleta A (UVA).
MÉTODOS: Amostras de S. aureus colocadas em uma placa de 96 poços (em triplicata) foram expostas a riboflavina 0,1% (B2) e luz ultravioleta (comprimento de onda de 365 nm) poder de 3 mW/cm2, 8 mm de diâmetro, por 30 minutos. Grupos controles foram também preparados em triplicata: controle branco, somente luz ultravioleta A, somente riboflavina e controle morto. A viabilidade bacteriana foi analisada usando microscópio de fluorescência. Para investigar a ocorrência de micro-organismos "viáveis porem não cultiváveis" a viabilidade celular foi avaliada utilizando-se placas de meio de cultivo bacteriano. Analise estatística foi realizada utilizando-se os valores obtidos em triplicata de cada grupo experimental.
RESULTADOS: Nenhuma diferença foi observada entre o grupo tratamento e os grupos controle (p=1).
CONCLUSÃO: A combinação riboflavina 0,1% e luz ultravioleta 365 nm de comprimento de onda não demonstrou atividade antimicrobiana contra S. aureus oxacilina sensível.
Descritores: Ceratite; Riboflavin; Úlcera da córnea; Staphylococcus aureus; Raios ultravioleta; Reagentes para ligações cruzadas
INTRODUCTION
Microbial keratitis is a sight-threatening disease due to injury or trauma of corneal surface, and predisposing factors include poor contact lens hygiene(1). Successful treatment requires prompt characterization of the causative agent and application of specific chemotherapeutic therapy(2,3). Since the introduction of sulfonamides and penicillin in the treatment of bacterial keratitis, an evolution in therapy has been observed(3,4). However, virulence factors have decreased effectiveness of antibiotic monotherapy. One possible reason to explain the occurrence of antibiotic-resistant bacteria could be the selection of clones related with the increasing resistance factors faced on the development of newest and broad range aggressive therapeutic profiles. In addition, delay in the selection of appropriate antibiotic patterns may represent limiting factors of therapy and/or could provide development of bacterial resistance mechanisms(5.6).
Bacterial keratitis due to Staphylococcus aureus is an increasingly common ocular infection(7). Virulence factors of bacterium involve expression of exotoxins and subversion of neutrophil-mediated host defense system(8), which can result in severe inflammation, pain, corneal perforation, scarring, and loss of visual acuity. In general, staphylococcal resistance apparatus against antimicrobial agents include both intrinsic physical and genetic factors, i.e. cell-wall thickness due to peptidoglycan synthesis and gene expression on a specific staphylococcal cassette chromosome, respectively(9,10).
The occurrence of antibiotic-resistant bacteria, with emphasis in staphylococcal infection, has prompted ophthalmologists to study the antimicrobial activity of additional biological, chemical and physical sources as adjunctive or alternative therapies for bacterial keratitis. Given that, UV-radiation can cause biosynthesis failures leading to cell death(11), the application of long-wavelength ultraviolet light (UVA) associated with vitamin B2 (riboflavin), as a photosensitizer, can emerge as an alternative tool for inactivation of bacterial pathogens. Such therapy, has been proposed for inactivation or reduction of the amount of bacterial pathogens from blood components and corneal tissue(12,13).
In order to provide information concerning a potential therapeutic effect on Gram-positive bacterial keratitis, the antimicrobial effect of UVA light in association with B2 against S. aureus strain was evaluated.
METHODS
Staphylococcus aureus strain
All standard reference strains assayed by antimicrobial susceptibility tests in vitro were derived from S. aureus (SA) ATCC 29213. The bacterium was grown for 24h at 37ºC in Nutrient broth medium (Difco, Franklin Lakes, NJ, USA) and the turbidity of cell concentration was adjusted to match a no. 0.5 McFarland optical density standard. The bacterial solution used in all experiments in vitro was standardized at concentration of approximately 108 colony forming units (CFU)/ml.
In vitro assays
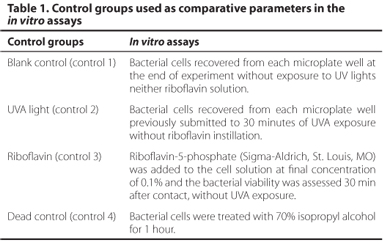
In order to provide comparative parameters of the antibacterial effect of UVA light and riboflavin in S. aureus, four control groups were performed as showed in table 1. Each control group was assayed in triplicate.
In the experimental group, 40 µl of bacterial solution was homogenized with B2 solution 0.1% and placed into each well of a sterile 96-well microplate lid (Corning Life Science, Lowell, MA, USA). Each well of the microplate lid has internal diameter of 7.85 mm, thus ensuring that the entire area can be exposed to UV light spot diameter of 8 mm, in a thin layer. Homogenized solution of S. aureus and riboflavin was exposed to UVA light radiation provided by a dedicated source (Opto Xlink, Opto, São Carlos, SP, Brazil) delivering wavelengths of 365 nm and irradiance of 3 mW/cm2, for 30 min. After the UVA light exposure the homogenized sample was recovered from each well and the bacterial viability was assayed by using the LIVE/DEAD BacLight bacterial viability staining kit (Molecular Probes Inc. , Eugene, OR, USA). Briefly, 5 µl of bacteria/ riboflavin irradiated mixture was homogenized with two fluorescent nucleic acid stains (SYTO9 and propidium iodide). The solution was placed onto a glass slide, covered with a coverslip and sealed. Bacteria were visualized under an Olympus IX81 inverted microscope (Olympus America Inc. Center Valley, PA, USA) equipped with filter packs GFP3035B and Texas Red 4040B (Semrock, Inc. Rochester, NJ, USA). The fluorescent digital images were captured by a Hamamatsu Photonics C9100-02 EMCCD camera (Hamamatsu Photonics, Japan), at a magnification of 40x. The red and green fluorescence was measured using the Imaris software (Bitplane Inc, South Windsor, CT, USA).
The leftover mixture of bacteria/riboflavin, was diluted in sterile saline solution (0.85%) to reach a final volume of 100 µl. After homogenization this suspension was inoculated onto a blood-sheep agar plate (BD Franklin Lakes, NJ, USA) by a spread plate technique. After incubation at 37ºC for 24h in an ambient-air incubator, the bacterial growth was evaluated qualitatively; assuming that each viable bacterium in suspension should form an individual colony on the culture medium. Both microscopic and plate-culture procedures in the experimental group were done in triplicate.
Statistical analyses
For statistical analyses, the mean value of all three measurements was calculated and compared among groups with the Kruskall-Wallis test. Bonferroni method was used to correct for multiple comparisons. A p-value <0.05 was considered statistically significant. All analyses were done with Stata v. 10 (College Park, Texas, USA).
RESULTS
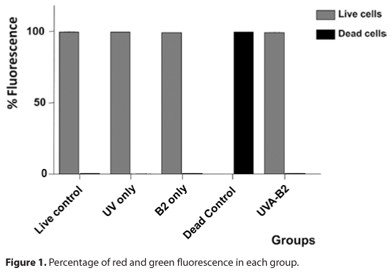
Antibacterial properties of UVA light and riboflavin were assayed by both microscopic and plate culture methods. The SYTO® 9 green-fluorescent nucleic acid stain labels all bacteria; those with intact membranes and those with damaged membranes. On the other hand the propidium iodide labels in red only bacteria with damaged membrane, causing a reduction in the green stain fluorescence when both dyes are present. The only group who presented cells stained in red, demonstrating damaged cells membrane, were the samples treated with 70% isopropyl alcohol, with 100% red fluorescence. (Figure 1) All the other groups (live control, only UVA, riboflavin alone (B2) and UVA-B2) revealed viable cells stained in green fluorescence. The mean green fluorescence percentage was 99.8%, 99.8%, 99.6% and 99.7%, respectively for the blank control, only UVA light, only riboflavin and UVA-B2 respectively (Figure 1). There was no statistical difference in the amount of green fluorescence among the groups.
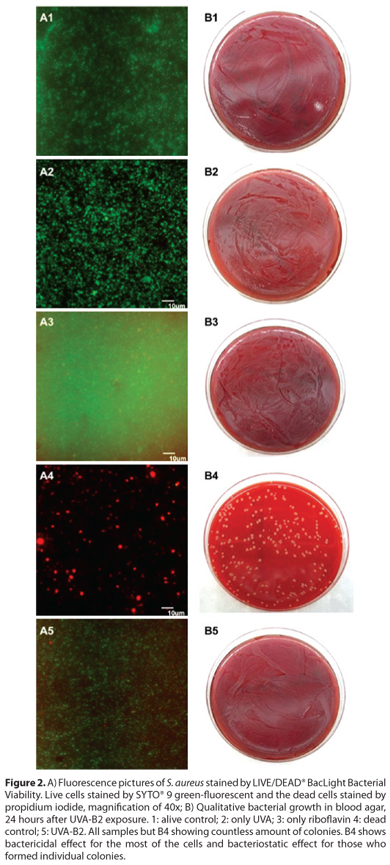
The results shown in figure 2 reveal neither bactericidal nor bacteriostatic activities in control groups 2 and 3, which evaluated the individual effect of UVA and B2 in the cell viability of S. aureus, respectively. In addition, control group 4, which represented the dead control, showed bactericidal effect for the most of the cells and bacteriostatic effect for those who formed individual colonies (Figures 2, A4 and B4). In the experimental group, which evaluated the combined antibacterial effect of UVA light and riboflavin, the S. aureus proved to be resistant to this physical-chemical technology under the parameters used in our experiments. Figures 2-A5 and 2-B5 show a countless amount of viable bacteria. This corroborates the results obtained from the individual application of UVA and riboflavin, which was demonstrated neither bacteriostatic nor bactericidal effect in S. aureus.
DISCUSSION
The rationale for use of UVA light associated with vitamin B2 for infectious keratitis treatment is based mainly on the riboflavin-based pathogen reduction technology (PRT) used in the microbial disinfections process of blood products(14,15). However, the UVA-B2 procedure designed to therapeutic application on keratoconus, which was evaluated in this study, uses UVA light wavelength of 370 ± 5 nm and total irradiance dose of 3.4 J based on safety studies(16). In comparison the riboflavin-based pathogen reduction technology (PRT) delivers higher total energy (6.2 J) as well as a shorter wavelength (265 to 370 nm)(17), which could causes more damage to the cells and DNA of pathogen, providing effective action in the bacterial death. Basically, the infectivity of pathogens is reduced by three complementary procedures: (1) the direct damage of nucleic acids of the pathogens by the UVA light, (2) the damage of proteins and membranes of microorganisms by reactive oxygen species generated when riboflavin absorbs light and interacts with dissolved oxygen in solution and (3) the damage of genetic machinery by the interaction of riboflavin with microbial nucleic acids(18,19).
The 'gold-standard' method, which is proposed to determine the microbial viability, is the growth of colonies on a nutrient agar surface after a period of incubation under a specific temperature and atmospheric conditions rates (20). The plate count method is based on the premise that each viable bacterium can grow, divide and become a colony, via binary fission in a suitable growth medium. Thus, unlike bacterial counts by the method of direct microscopy in which all cells (dead and alive) are counted, the plate culture procedure allows qualitative and quantitative analysis of viable cells in a sample. However, in the experimental protocols involving nutrient deprivation, environmental stress or antimicrobial effect of biological, chemical and/ or physical agents the cultivation method can fail to assess the cell viability if the target pathogen enters into the metabolic dormant stage of "viable but non-culturable" (VBNC)(21,22). In general, bacteria in the VBNC state are not able to grow on the artificial cuture media, but they are alive and capable of renewed metabolic activity(20). One of the approaches to determine the VBNC state is establishing the presence of an intact cytoplasmic membrane. For this reason, a methodological approach based on additional testing of fluorescent dye to study the bacterial viability was proposed in this study. The SYTO® 9 green-fluorescent nucleic acid stain labels all bacteria those with intact membrane and those with damaged membrane, while the propidium iodide labels in red only bacterial with damaged membrane, causing a reduction in the green stain fluorescence when both dyes are present(23).
In a previous study, Martins et al. (24), demonstrated no bacterial growth in a suitable media 24h after UVA-B2 exposure. However, a hypothesis that leads the cells to enter in a VBNC metabolic state could be the presence of free radicals(21). Some authors(25) suggested that non-growing cells might produce free radicals on exposure to high nutrient-containing medium, which might prevent cell division and the origins of new colonies. The non bacterial growth after UVA-B2 exposure observed in that experimental study could be justified by the presence of production of singlet oxygen, and generation of hydrogen peroxide. Thus, those bacterial cells assayed under the UVA-B2 exposure might not be in fact dead, but instead in a VBNC state.
The main goal of the study was first observe the efficacy of the combination UVA-B2 as a bactericidal agent. We were able to demonstrate the bacterial viability only by using the fluorescent dyes as done in previous studies(11,21). Thereafter, we did not consider to count the exact amount of colonies using the traditional dilution methods, instead, the objective of placing the leftover treated sample and the control groups onto a plate culture were merely illustrative, showing that the groups stained in green were able to grow in a plate culture, meanwhile the group stained mostly in red only a few cells were able to grow in the plate culture. Curve response was not performed because a safety protocol previous established for this technology was followed in order to avoid the occurrence of intra-ocular damages, mainly by the UVA light(16). Instead, we have tried higher concentrations of riboflavin, but riboflavin 0.5% and 1% in the presence of the fluorescence dyes caused a fast bleaching which preclude high quality fluorescence microscopic data.
In conclusion, we were able to demonstrate a qualitative agreement concerning the lack of efficacy of photodynamic therapy against S. aureus. However, the quantitative data concerning the agreement of antibacterial effect of components of photodynamic therapy was elusive due to staphylococcal resistance against both physical (UV light) and chemical (riboflavin) agents tested and, consequently, the occurrence of uncountable amount of bacteria into culture media. Finally, the current related that there was no bactericidal effect of UVA/B2 settings used in treatment of keratoconus, against oxacillin susceptible S. aureus.
REFERENCES
1. Green M, Apel A, Stapleton F. Risk factors and causative organisms in microbial keratitis. Cornea. 2008;27(1):22-7.
2. Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. Br J Ophthalmol. 2001;85(7):842-7.
3. Oliveira AD, D'Azevedo PA, Francisco W. In vitro activity of fluoroquinolones against ocular bacterial isolates in São Paulo, Brazil. Cornea. 2007;26(2):194-8.
4. Thygeson P, Spencer WH. The changing character of infectious corneal disease: emerging opportunistic microbial forms (1928-1973). Trans Am Ophthalmol Soc. 1973;71:246-53.
5. Bertino JS Jr. Impact of antibiotic resistance in the management of ocular infections: the role of current and future antibiotics. Clin Ophthalmol. 2009;3:507-21.
6. Kowalski RP, Dhaliwal DK. Ocular bacterial infections: current and future treatment options. Expert Rev Anti Infect Ther. 2005;3(1):131-9.
7. Dajcs JJ, Thibodeaux BA, Girgis DO, O'Callaghan RJ. Corneal virulence of Staphylococcus aureus in an experimental model of keratitis. DNA Cell Biol. 2002;21(5-6):375-82.
8. Hayashida AS, Amano S, Park PW. Syndecan-1 promotes Staphylococcus aureus corneal infection by counteracting neutrophil-mediated host defense. J Biol Chem. 2001;286(5):3288-97.
9. Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1(3):147-55.
10. Deurenberg RH, Stobberingh EE. The evolution of Staphylococcus aureus. Infect Genet Evol. 2008;8(6):747-63.
11. Berney M Hammes F, Bosshard F, Weilenmann HU, Egli T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl Environ Microbiol. 2007;73(10):3283-90.
12. AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, et al. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion. 2005;45(8):1335-41.
13. Moren H, Malmsjo M, Mortensen J, Ohrstrom A. Riboflavin and ultraviolet a collagen crosslinking of the cornea for the treatment of keratitis. Cornea. 2010;29(1):102-4.
14. Pelletier JP, Transue S, Snyder EL. Pathogen inactivation techniques. Best Pract Res Clin Haematol. 2006;19(1):205-42.
15. Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44(6):877-85.
16. Spoerl E, Mrochen M, Sliney D, Trokel S, Seiler T. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26(4):385-9. Comment in: J Refract Surg. 2012;28(2):91-2.
17. Goodrich RP, Edrich RA, Li J, Seghatchian J. The Mirasol PRT system for pathogen reduction of platelets and plasma: an overview of current status and future trends. Transfus Apher Sci. 2006;35(1):5-17.
18. Joshi P. Comparison of the DNA-damaging property of photosensitized riboflavin via singlet oxygen and superoxide radical mechanisms. Toxicol Lett. 1985;26(2-3):211-7.
19. Kumar V, Lockerbie O, Keil SD, Ruane PH, Platz MS, Martin CB, et al. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem Photobiol. 2004;80:15-21.
20. Davey HM. Life, death, and in-between: meanings and methods in microbiology. Appl Environ Microbiol. 2011;77(16):5571-6.
21. Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005;43 (Spec no):93-100.
22. Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34(4):415-25.
23. Barbesti S, Citterio S, Labra M, Baroni MD, NerI MG, Sgorbati S. Two and three-color fluorescence flow cytometric analysis of immunoidentified viable bacteria. Cytometry. 2000;40(3):214-8.
24. Martins SA, Combs JC, Noguera G, Camacho W, Wittmann P, Walther R, et al. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: a potential new treatment for infectious keratitis. Invest Ophthalmol Vis Sci. 2008;49(8):3402-8.
25. Bloomfield SF, Stewart GS, Dodd CE, Booth IR, Power EG. The viable but non-culturable phenomenon explained? Microbiology. 1998;144(Pt 1):1-3. Comment in: Microbiology. 1998;144(Pt 5):1131.
 Correspondence address:
Correspondence address:
Renata Tiemi Kashiwabuchi
Rua Botucatu, 820
São Paulo (SP) - 04023-062 - Brazil
E-mail: [email protected]
Submitted for publication: September 19, 2012
Accepted for publication: September 23, 2012
Funding: This study was supported by Research to Prevent Blindness Inc, NY, NY to the Wilmer Ophthalmological Institute to conduct this study (laboratory supplies).
Disclosure of potential conflicts of interest: R.T.Kashiwabuchi, None; Y.A.Khan, None; F.R.S.Carvalho, None; F.Hirai, None; M.S.Campos, None; P.J.McDonnell, None.
Study carried out at The Wilmer Ophthalmological Institute, The Johns Hopkins University, School of Medicine, Baltimore, Maryland, USA
CEP UNIFESP: 1498/11