INTRODUCTION
Pigment epithelial detachment (PED) is a pathological process in which the retinal pigment epithelium (RPE) separates from the underlying Bruch's membrane (BM) as a result of the presence of blood, serous exudate, drusen, or a neovascular membrane1,2. Drusenoid retinal pigment epithelial detachment (DPED) is a type of PED that evolves from confluent and large, soft drusen3,4, which are extracellular deposits that accumulate between the basal lamina of the RPE and the inner collagenous layer of BM5, and is considered to be a feature of non-neovascular age-related macular degeneration (AMD)6.
Historically, this fundus lesion has been distinguished from other PEDs, such as fibrovascular PEDs and hemorrhagic PEDs, by clinical appearance, fluorescein angiography (FA) findings, histopathology, and a better short-term visual prognosis4-8. In addition, DPEDs are associated with intermediate AMD, unlike other types of PED, which are generally associated with advanced neovascular AMD6.
DPED can lead to choroidal neovascularization (CNV)6,9. However, in the early stages of DPED-associated CNV, diagnosis may be diffi cult because spectral-domain optical coherence tomography (SD-OCT) may not show characteristic signs of CNV (OCT-silent CNV). In this situation, FA and indocyanine green angiography (ICGA) may be able to identify evidence of CNV10. We report two cases of DPED with OCT-silent CNV that responded to treatment with intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy.
CASE REPORTS
Case 1
A 69-year-old white man presented decreased central vision in the right eye for 5 days, 4 months after cataract surgery. The patient underwent radial keratotomy about 20 years previously, and had been informed, 9 years before undergoing cataract surgery, that he had drusen. His medical history was unremarkable.
Examination demonstrated a best-corrected visual acuity (BCVA) of 20/125 in the right eye and 20/40 in the left eye. Intraocular pressure was 12 mmHg in both eyes. Slit-lamp examination of the right eye revealed a tilted intraocular lens with the nasal haptic in the ciliary sulcus and the temporal haptic in the capsular bag. Dilated fundus examination demonstrated a grade zero epiretinal membrane (ERM) in the right eye, and multiple intermediate drusen without evidence of CNV in both eyes. SD-OCT (Spectralis SD-OCT; Heidelberg Engineering, Heidelberg, Germany) showed an ERM and a subfoveal double hump-shaped PED in the right eye (fovea centered 20º line, 512 A-scans per B-scan, automatic real-time setting of 15) (Figure 1) and a subfoveal DPED in the left eye. Late-phase FA of the right eye showed staining of the drusen and PED without any leakage, whereas ICGA demonstrated a hot spot in the location of the PED. In the left eye, FA demonstrated staining of the drusen, and the ICGA results were unremarkable. The right eye was treated with an intravitreal injection of ranibizumab 0.5 mg for a presumed low-flow choroidal neovascular membrane. At 6 weeks post-injection, the BCVA in the right eye had improved to 20/63, with no changes observed on OCT. Following two additional intravitreal injections of ranibizumab 0.5 mg (performed 6 and 12 weeks after the initial injection, res pectively), the BCVA had improved to 20/50 with no change observed on OCT. After the fourth injection, which was performed 18 weeks after the initial injection, improvements in both the BCVA and the anatomy were observed; the PED progressively decrea sed in size, leaving some hyperreflective foci above a normoreflective inner segment ellipsoid line, along with a nearly flat RPE on OCT after the fifth injection (given 8 weeks after the fourth injection). After the fifth injection, at 10 weeks, the BCVA had improved to 20/32. A sixth injection was administered, and the inter-injection interval after the fifth injection was extended to 12 weeks.
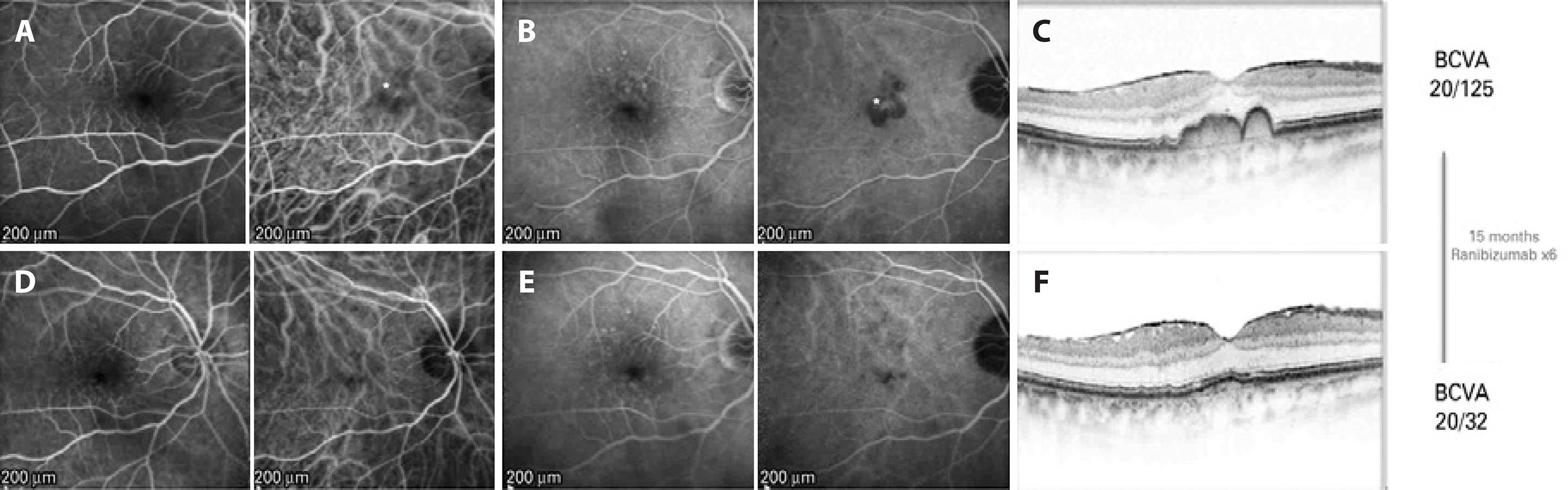
Figure 1 (Case 1): A) Early phase pre-treatment. Left: fluorescein angiography (FA) with discrete foci of parafoveal hypofluorescence as a result of drusen blockage. Right: indocyanine green angiography (ICGA) demonstrates a hyperfluorescent spot in the foveal region (*). B) Late phase pre-treatment. Left: FA shows staining of the drusen. Right: ICGA demonstrates increased hyperfluorescence and better delineation of the foveal lesion (*). C) Pre-treatment optical coherence tomography (OCT) demonstrates an epiretinal membrane and a double-humped PED. D) Early phase post-treatment. Left: FA shows early hyperfluorescence from the drusen. Right: ICGA no longer shows the hyperfluorescent lesion. E) Late phase post-treatment. Left: FA shows staining of the drusen. Right: ICGA demonstrates absence of the hot spot visualized on the pre-treatment ICGA. F) Post-treatment OCT shows that the PED has flattened.
Case 2
A 79-year-old white man presented after 15 days of decreased central vision in the left eye 1 month after cataract surgery in that eye. He had smoked cigarettes for over 30 years, and denied other ocular surgeries, pathologies, and medication use.
Examination demonstrated a BCVA of 20/32 in the right eye and 20/100 in the left eye. Intraocular pressure was 16 mmHg in the right eye and 14 mmHg in the left eye. Slit-lamp examination was notable for a nuclear sclerotic cataract in the right eye and a well-positioned posterior chamber intraocular lens in the left eye. Dilated fundus examination exhibited irregular foveal pigmentation with medium-sized drusen in the right eye and a foveal DPED in the left eye. FA showed a hyperfluorescent point inferonasal to the fovea in the left eye that increased in the late phases of the FA, and did not correspond to the site of the DPED (Figure 2). ICGA showed a hypo fluorescent dot in the region of the PED and normal retinal and choroidal vasculature. SD-OCT (hyperfluorescent lesion centered 20º line, 512 A-scans per B-scan, automatic real-time setting of 15) findings were consistent with the fundus examination findings. Based on the FA findings, an inferonasal CNV was suspected, and the patient was treated with three intravitreal ranibizumab 0.5 mg injections administered at 6-week intervals. The lesion gradually faded throughout the treatment course, leaving a flat BM with a disrupted external retina on OCT; the BCVA 8 weeks after the third injection was 20/40. At this time, a fourth injection was administered, and the BCVA improved to 20/32 12 weeks after.
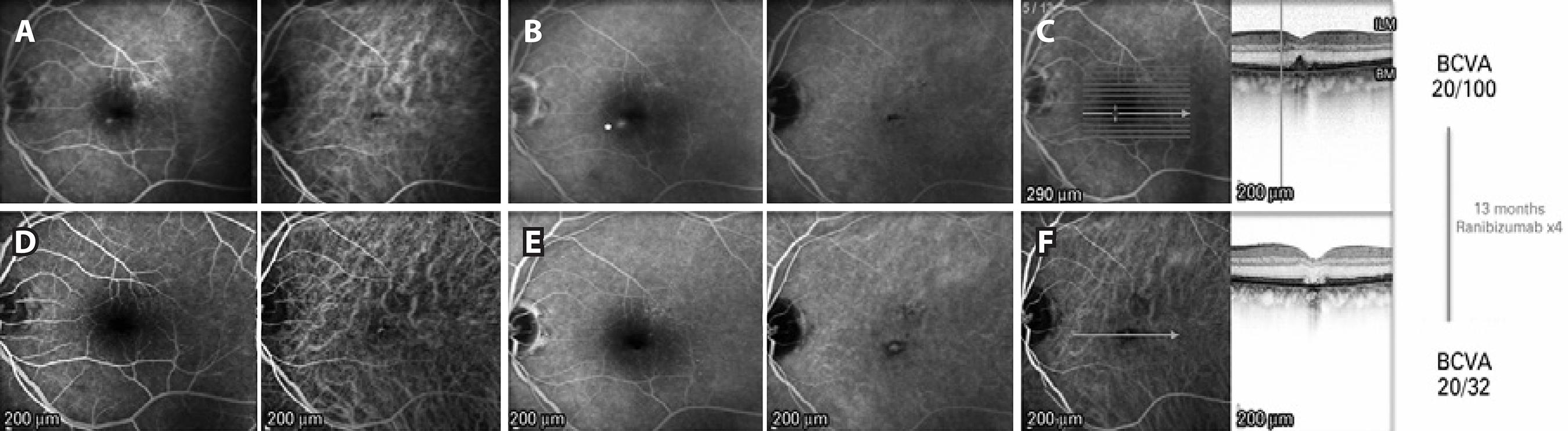
Figure 2 (Case 2): A) Early phase pre-treatment. Left: FA shows a small round hyperfluorescent lesion inferonasal to the fovea. Right: ICGA demonstrates a hypofluorescent foveal dot in the PED. B) Late phase pre-treatment. Left: FA shows an increased area of fluorescein leakage from the lesion. Right: ICGA demonstrates no late changes. C) Pre-treatment multimodal FA/OCT. Of note, the hyperfluorescent dot observed on FA does not correspond to the foveal PED. D) Early phase post-treatment. Left: FA shows absence of the hyperfluorescent dot seen on the pre-treatment FA. Right: ICGA demonstrates transmission hyperfluorescence. E) Late phase post-treatment. Left: No late hyperfluorescent dot is observed on FA. Right: ICGA demonstrates transmission hyperfluorescence. F) Post-treatment FA/OCT demonstrates involution of the PED with atrophy of the outer retina.
DISCUSSION
DPED is part of the clinical spectrum of AMD5,6. A postulated me chanism of the development of DPED with a small accumulation of fluid without the classical signs of CNV is that the confluence of soft drusen is associated with a buildup of lipidic membranous debris that creates a hydrophobic barrier in BM, resulting in the accumulation of fluid and enlargement of the DPED11.
We have described two patients with DPED without the characte ristic signs of CNV on OCT, whose vision improved after anti-VEGF therapy. FA and ICGA examination revealed suspected CNV without definitive evidence, which is probably due to low-flow CNV. Evidence for this diagnosis was found after prompt clinical response, which resulted in improved visual acuity following anti-VEGF therapy, being less likely to be related to the natural involution of the PEDs.
Interestingly, both cases occurred after cataract surgery, which may have served as an inflammatory stimulus for CNV development. No previous studies have reported that cataract surgery can increase the conversion rate from dry to wet AMD12,13; however, in patients that already had CNV, the number of injections tended to increase14. It is possible that silent CNV was already present in our patients and that their development was triggered by surgical intervention.
The current report is the first to demonstrate the anatomical and functional responsiveness of OCT-silent CNV to an anti-VEGF agent in the setting of DPED with progressive worsening of vision. Type 1 CNV without exudative findings have been demonstrated in asymptomatic patients. Roisman et al. and Querques and Souied looked for CNV in the eyes of asymptomatic patients with intermediate AMD using OCT angiography15,16, and found neovascular nets in all eyes with plaques on ICGA. Nevertheless, because no worsening of vision was seen, the question was raised whether we should treat these lesions, even with evidence of CNV growth16.
In these scenarios of vision loss and suspected neovascularization in dry drusenoid lesions, FA and ICGA still appear to have roles in the diagnosis and follow-up of AMD, and anti-VEGF therapy may be a potential therapeutic option. New technologies such as OCT angiography17, which was not yet available at the time that these patients were treated, may provide additional information regarding the early signs of CNV in patients with AMD, thus enabling earlier diagnosis.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

