INTRODUCTION
Conjunctival and limbal tumors comprise a wide variety of conditions from benign to malignant lesions that may threaten the visual function and life of the patient. The most common types of ocular surface tumors are squamous cell carcinoma (SCC), primary acquired melanosis (PAM), lymphoma, and malignant melanoma1. Older age, male gender, prolonged ultraviolet B exposure, and human papilloma virus are the risk factors for developing ocular surface squamous neoplasia (OSSN). These neoplasms are classified as benign, pre-invasive, or invasive. Benign OSSN includes papillomas, pseudoepitheliomatous hyperplasia, and benign hereditary intraepithelial dyskeratosis. Pre-invasive lesions, such as conjunctival intraepithelial neoplasms (CIN) grade I-III, have malignant potential and are classified as mild, moderate, or severe depending on the degree of involvement of the dysplastic epithelium. Mild dysplasia or CIN grade I is defined as dysplasia of the lower third of the conjunctival epithelium; dysplasia that extends into the middle third is defined as CIN grade II; and dysplasia that extends into the upper third is defined as CIN grade III or carcinoma-in situ2. Invasive OSSN consists of SCC and, rarely, mucoepidermoid carcinoma. These lesions were traditionally treated by complete surgical excision with or without adjunctive therapy to reduce the rate of recurrence1. Complete resection and histopa thological control of the borders are very important3.
The amniotic membrane (AM), which is the innermost layer of the placenta, contains a thick basement membrane and an avascular stroma, which may promote epithelial cell proliferation4. The activity of the AM has been attributed to various mechanisms, most of which are related to the structural and biochemical composition of the membrane; however, these have not been proven. The basement membrane characteristics of the AM are similar to that of the cornea and conjunctiva5. AM transplantation (AMT) promotes epithelial wound healing and exerts potent anti-inflammatory and anti-scarring effects on the ocular surface. These valuable properties make the AM an ideal tissue for reconstruction of ocular surface tumors6-9. Several studies have been performed for ocular surface reconstruction after OSSN removal. Asoklis et al. reported that AMT was an effective technique without complications after removal of conjunctival and limbal tumors in nine patients9. Palamar et al. studied the long-term efficacy of AMT for ocular surface reconstruction in 21 OSSN patients. They found that AMT was an effective procedure even for tumors larger than 10 mm10.
Cryotherapy has been used as an adjunctive therapy to reduce the recurrence rates. Surgical excision with wide margins had not been sufficient in all cases. Occasionally, a surgeon can miss the tumor cells at the margins or base of the sclera. Cryotherapy destroys these small groups by superficial and deep freezing techniques and reduces recurrence rates11. The reported recurrence rate among patients who underwent cryotherapy after surgical excision ranged from 7.1% to 12.3%11-13. In this paper, we reported our long-term experience on AMT and cryotherapy following excision of large conjunctival and limbal tumors.
METHODS
This study was approved by the institutional ethics committee and was conducted in accordance with the Declaration of Helsinki. The details of the study were explained to the patients and written informed consent was obtained. Fourteen patients who underwent AMT procedure and cryotherapy after excision of limbal and conjunctival tumors were analyzed. All surgeries were performed by one surgeon (YK) at a single center. Histopathologic analyzes were done at the pathology department of the same hospital.
During all visits, slit lamp bio-microscopy and fluorescein staining were performed. Any complications, such as scarring, vascularization, corneal opacities, and recurrences, were noted. Complete healing without any complication was defined as a successful; non-complete healing without recurrence was defined as partial success; and recurrence was defined as failure.
Excision of the tumors
All surgeries were performed under sedation or local anesthesia with 1:1 mixture of 2% lidocaine and 0.5% bupivacaine. The tumors were successfully excised completely with lesion-free margins mea suring 2-3 mm. The surgeon avoided any tumor contact with the surgical instruments to prevent tumor seeding into the other sites. Fresh instruments were used after removal of the tumor. The management of lesions in the limbal area involved alcohol epitheliectomy for the corneal component, with wide margins for the conjunctival component followed by freeze-thaw cryotherapy to the remaining adjacent bulbar conjunctiva.
Amniotic membrane transplantation
After obtaining from donors who underwent elective caesarean sections, AMs were processed and stored in a solution of glycerine and Dulbecco's Modified Eagle Medium (DMEM) in a 1:1 ratio containing antibiotics at -80 ºC. Donor serums were checked for micro biologic safety at the time of donation and after six months.
A single layer of AM was placed over the defect area as stromal face down and was sutured with 8/0 vicryl one by one. No bandage contact lens was used to cover the AM. Postoperatively, all patients received fluorometholone, ofloxacin, and dextran 70 hydroxypropyl methylcellulose four times daily for one month. In three patients, sutures were removed on the fifteenth day postoperatively.
RESULTS
Of the 14 cases included in this study, 6 were men and 8 were women; mean age was 56 years (range, 15-82 years). All tumors were primary cases and did not receive any prior treatment. These excised tumors were classified as CIN (n=7), conjunctival nevus (n=5), PAM (n=1), and SCC (n=1). There was no local invasion to the orbit or local lymph node metastasis at the time of surgery. The bulbar conjunctiva was involved in all cases, the limbus was involved in 10 cases, and the cornea was involved in 6 cases. Table 1 presents the size, localization, pathologic diagnoses, and additional surgical therapy of the study population. The average measurement of the tumor base was 14.8 mm (range 6-20 mm, SD 16 mm). The mean follow-up time period was 17.5 months (range, 6-60 months, SD 20 months).
Table 1 Surgical characteristics of the excised tumors
| Case | Tumor eye localization base (conjunctival) | Limbal involvement | Corneal involvement | Pathologic diagnosis | Additional surgery |
|---|---|---|---|---|---|
| 01 | 10 × 15 LE superior nasal bulbar | + | - | N | - |
| 02 | 15 × 10 × 5 RE Nasal bulbar | + | + | CIN | - |
| 03 | 10 × 10 RE superior nasal bulbar | + | - | N | - |
| 04 | 20 × 20 RE nasal bulbar | + | + | CIN | + |
| 05 | 20 × 20 LE superior-nasal inferior bulbar | + | + | CIN | + |
| 06 | 10 × 15 RE nasal bulbar | + | + | CIN | - |
| 07 | 20 × 15 RE superior lateral bulbar | + | + | CIN | + |
| 08 | 10 × 15 LE inferior lateral bulbar | - | - | PAM | - |
| 09 | 15 × 10 LE lateral bulbar | + | + | SCC | - |
| 10 | 15 × 10 RE nasal bulbar | + | - | CIN | - |
| 11 | 3 × 3 RE nasal bulbar | - | - | N | - |
| 12 | 5 × 10 LE lateral bulbar | + | - | CIN | - |
| 13 | 2 × 2 RE nasal bulbar | - | - | N | - |
| 14 | 5 × 6 RE nasal bulbar | - | - | N | - |
RE= right eye; LE= left eye; N= nevus; CIN= conjunctival intraepithelial neoplasia; PAM= primary acquired melanosis; SCC= squamous cell carcinoma.
Table 2 shows the complications, recurrence, and cosmetic outcomes of the study. Complete healing was achieved in eight eyes. Limbal cell deficiency developed in two eyes. Figures 1-4 demonstrate the preoperative and postoperative images of cases with conjunctival tumor, SCC, and CIN. Four cases developed recurrence and were treated with the same surgical and medical procedures; these cases were noted as failure because of the development of superficial peripheral vascularization and corneal scar on follow-up after recurrence. Eight cases were reported as successful and two cases were reported as partial success.
Table 2 Characteristics of the cases on follow-up
| Case | Follow-up Pre-op Final (month) BCVA BCVA | Complications | Recurrence | Cosmetic appearance |
|---|---|---|---|---|
| 01 | 6,00 1.00 1.00 | - | - | Good |
| 02 | 12,00 0.70 0.70 | - | - | Good |
| 03 | 60,00 0.70 0.70 | - | - | Excellent |
| 04 | 6,00 0.30 0.30 | - | + | Excellent |
| 05 | 36,00 0.05 0.001 | Limbal insufficiency | + | Bad |
| 06 | 24,00 0.05 0.05 | Limbal insufficiency | - | Bad |
| 07 | 60,00 0.06 0.06 | Vascularization | + | Bad |
| 08 | 6,00 1.00 1.00 | - | - | Excellent |
| 09 | 6,00 0.06 0.06 | Corneal opacification | - | Bad |
| 10 | 6,00 0.50 0.50 | - | - | Excellent |
| 11 | 6,00 1.00 1.00 | - | - | Excellent |
| 12 | 6,00 1.00 1.00 | - | - | Good |
| 13 | 6,00 1.00 1.00 | Scar | + | Good |
| 14 | 6,00 1.00 1.00 | - | - | Excellent |
BCVA= best corrected visual acuity.
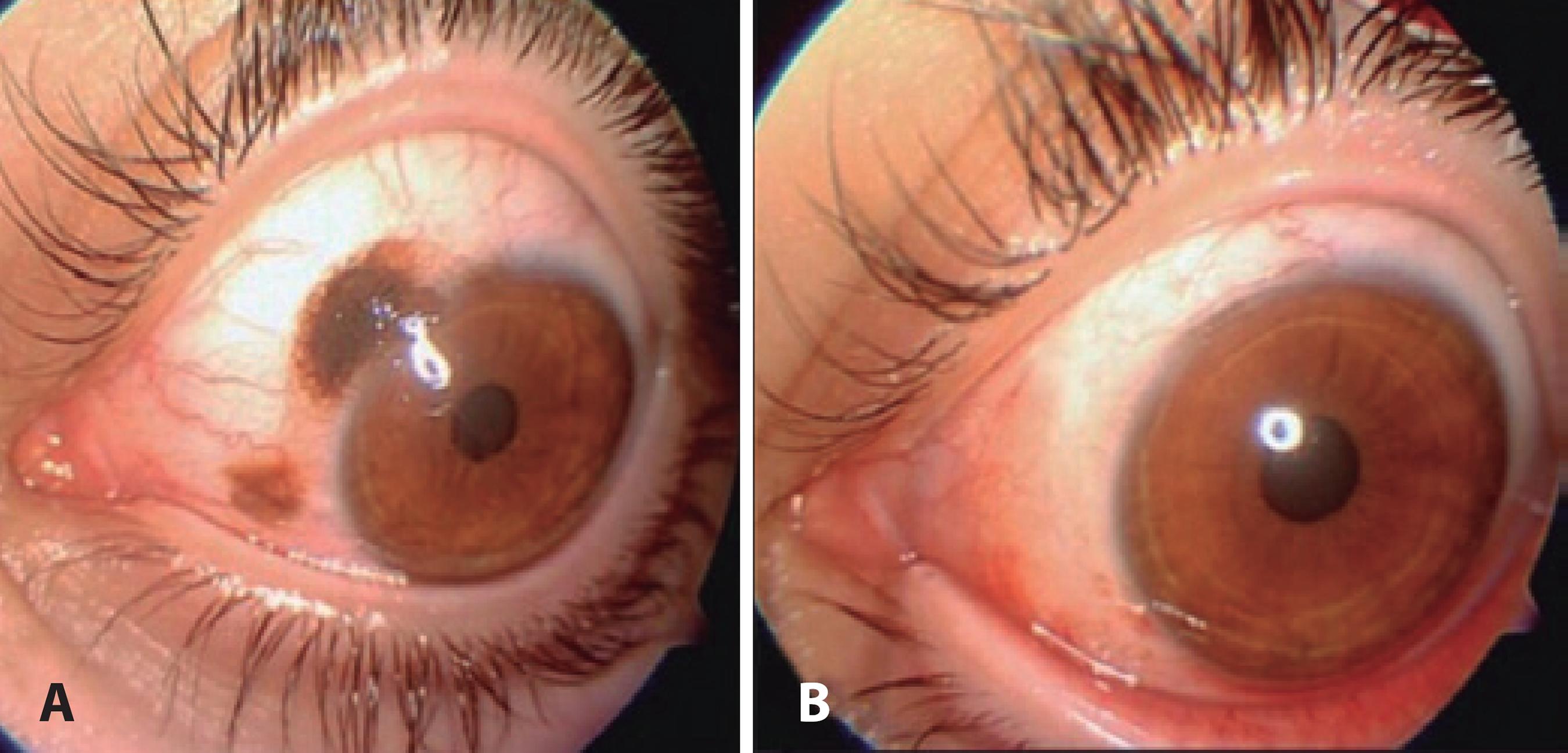
Figure 1 A superior-medial conjunctival nevus case. A) Preoperative and B) postoperative appearances.
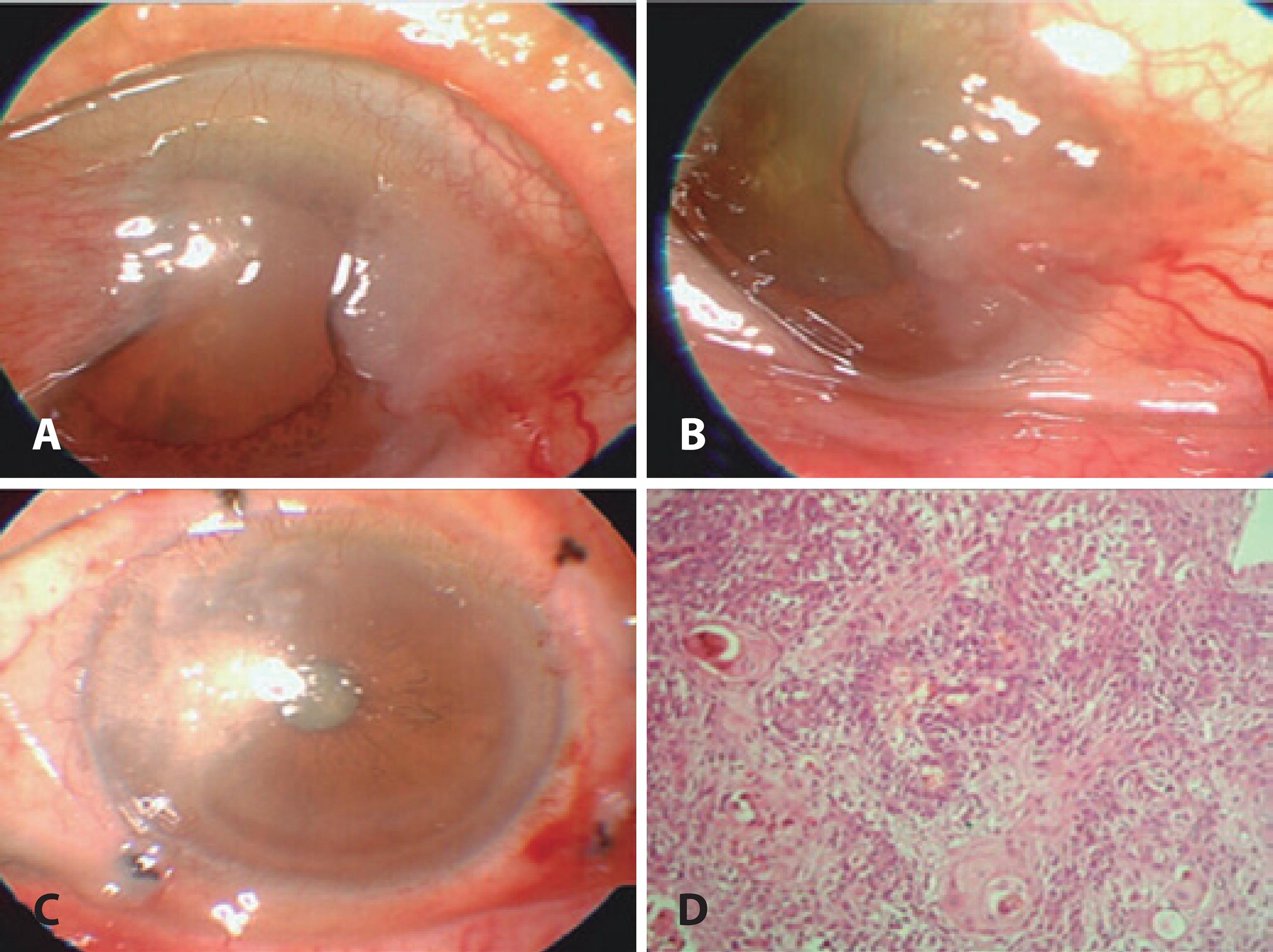
Figure 2 A and B) An SCC case involving the lateral bulbar conjunctiva and cornea. C. Excision of tumor with “no touch technique,” AMT is applied to the defect area and freze-thaw cryotherapy is applied to the free conjunctival margins. D) Histopathologic slide of SCC stained with hematoxilin-eosin.
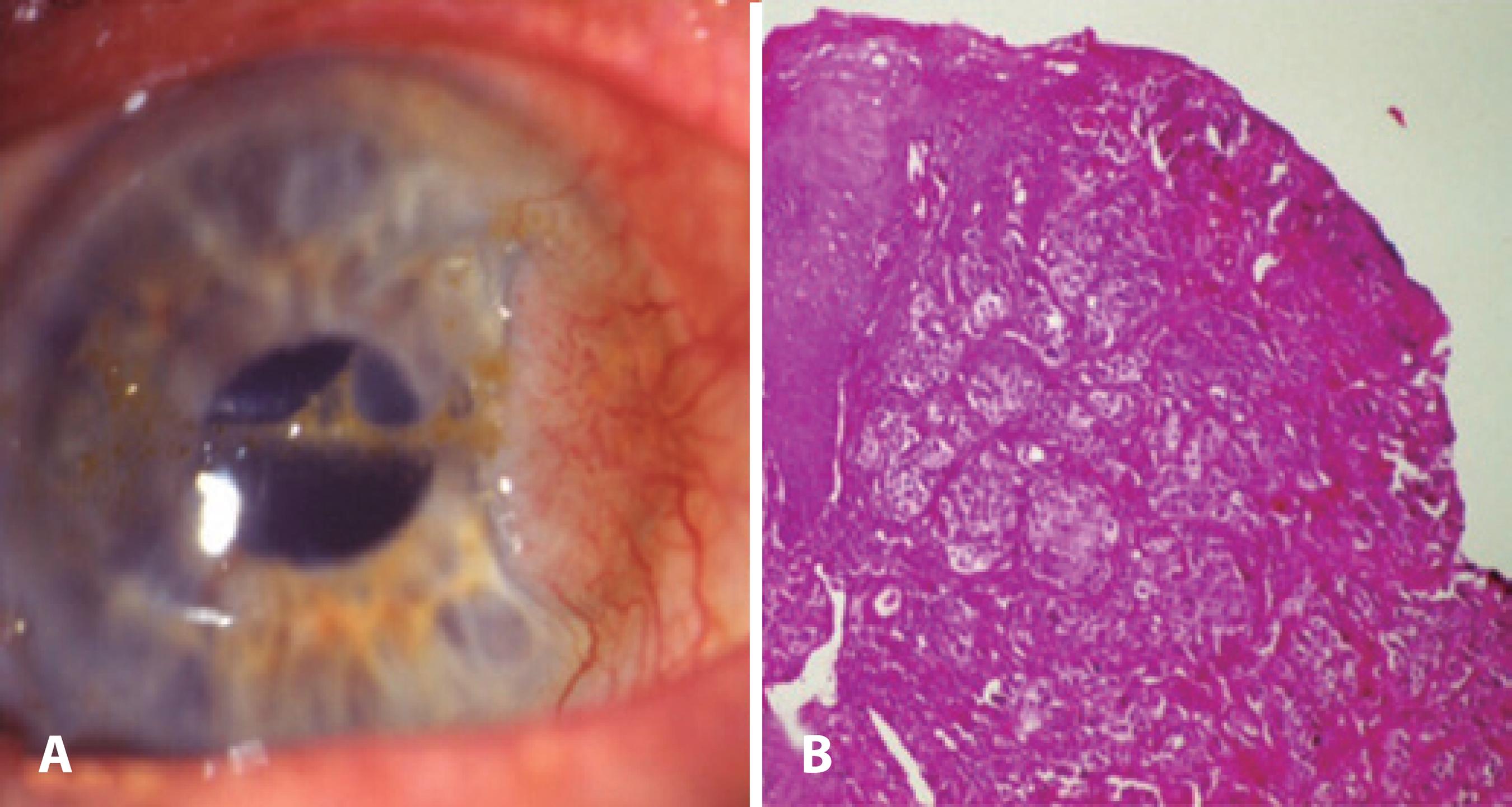
Figure 3 Excision of a CIN tumor with “no touch technique.” AMT is applied to the defect area and freze-thaw cryotherapy is applied to the free conjunctival margins. B) Histopathologic slide of CIN stained with hematoxilin & eosin.
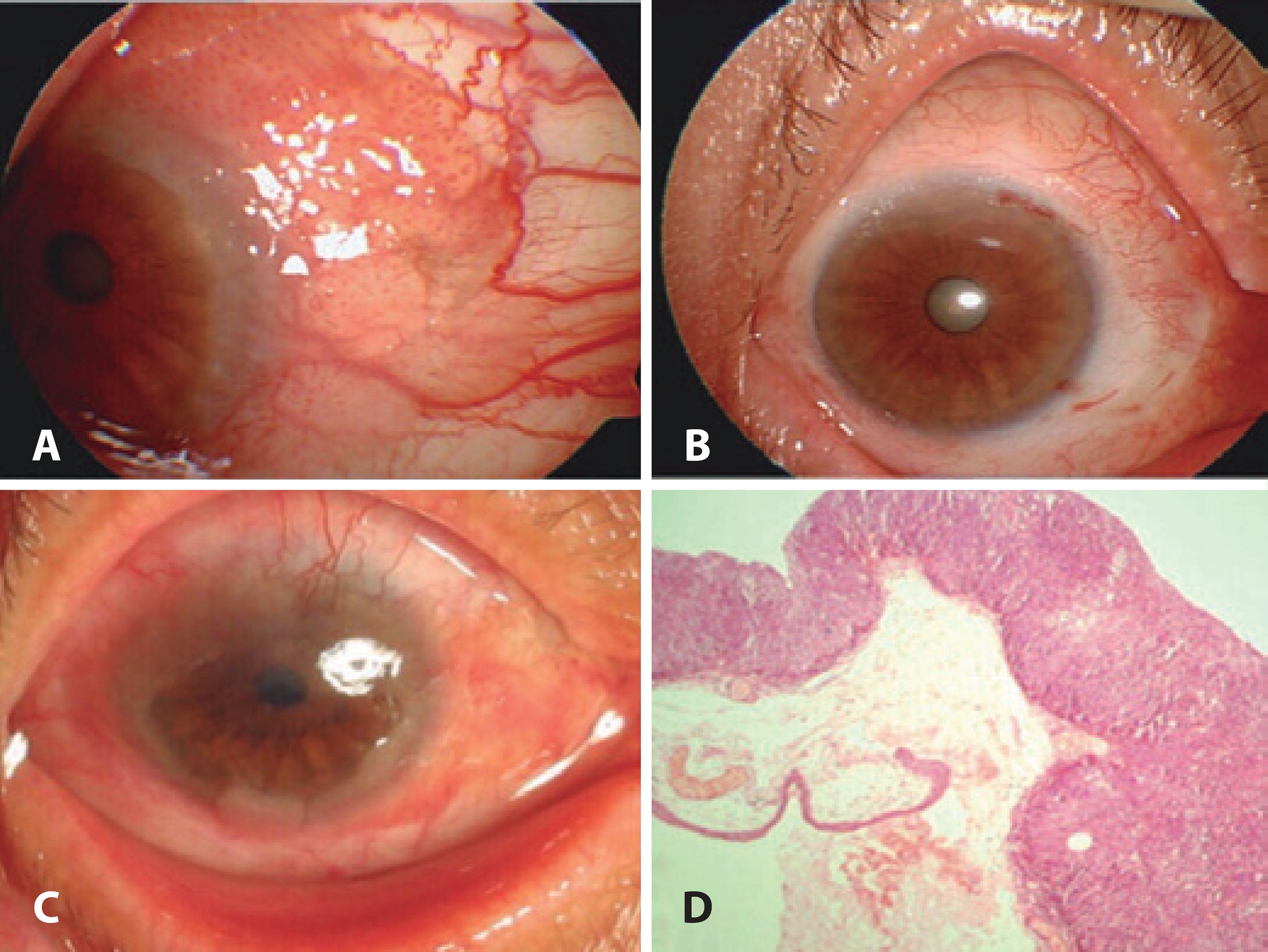
Figure 4 A) A CIN case involving the superior and medial bulbar conjunctivae and cornea. B) Excision of the tumor with “no touch technique.” AMT is applied to the defect area and freze-thaw cryotherapy is applied to the free conjunctival margins. C) Six months after the first surgery, there was recurrence and limbal deficiency. D) Histopathologic slide of CIN.
DISCUSSION
Management of conjunctival and limbal tumors is a significant therapeutic challenge. The key point in treating ocular surface tumors depends mainly on complete surgical removal1 because the presence of residual tumor cells in adjacent tissues might cause recurrence. Impression cytology (IC) has an important role in therapeutic monitoring of residual cells after tumor removal. Although this test cannot substitute histopathological evaluation, it is safe, simple, and non-invasive, particularly in cases that require multiple repeated biopsies. With this technique, a cellulose acetate paper is applied to the conjunctiva, cornea, and limbal region to obtain superficial cells. IC has been widely used for diagnosis of diseases; evaluation of sequential changes in the conjunctival, limbal, and corneal surfaces; and follow-up after treatment14.
Success of the treatment specifically rests on eradication of tumor cells from the ocular surface1,15. Therefore, complete removal should be accompanied by excision of a tumor-free margin, which measures 3-4 mm. Several adjunctive therapeutic measures, such as cryotherapy, mitomycin C (MMC), 5-fluorouracil, and interferon, have been used to reduce recurrence rate2,16. MMC has significant advantages over excision by treating tumor cells in the rapid cycle, but not the slow-cycle stem cells17. More recently, Hanada et al. studied eight cases to evaluate the efficacy of AMT combined with 0.04% MMC for reconstruction of conjunctival defects created during the OSSN removal; they found that complete healing can be achieved without any clinically significant complications18. However, previous studies reported serious complications, such as scleral melting, limbal stem cell deficiency, cataract, and infection19. In our study, we used cryotherapy to avoid these serious complications of MMC. Although corneal edema, intraocular inflammation, and fibrosis have been reported with cryotherapy, we did not encounter such complications in our cases.
Aside from the complications of adjunctive therapies, complications caused by surgical removal of tumors are frequently seen; these include symblepharon, corneal scarring, granulation tissue formation, and partial or total limbal stem cell deficiency20. Symblepharon is the most common complication that requires a large conjunctival flap or buccal mucosal graft to reconstruct the ocular surface21. The main concern in these challenging situations is to find a suitable technique for reconstructing the resected areas. Conjunctival, nasal-buccal mucosal grafts, and AM can be used for reconstruction of a large conjunctival surface after removal1,2. AM has several superior features over mucosal grafts. The use of thicker mucosal grafts has cosmetic disadvantages compared with the use of AM, particularly when visible areas of conjunctiva are involved in the surgery22. Furthermore, these thicker mucosal grafts might hide tumor growth for a long time. On the other hand, the use of AM allows surgeons to observe for recurrence of tumors underneath its transparent structure. AM also has the advantage of avoiding donor site morbidity, as may often be seen in patients in whom their own mucosal autografts were used22. Neuhaus et al. encountered donor site complications, such as submucosal scarring with contracture, after full-thickness mucous membrane grafting. Conjunctival autografting can also result in postoperative complications23. Vrabec et al. reported two cases of subconjunctival fibrosis at the graft harvest site following pterygium excision; one of them formed scar tissue that impaired extraocular mo vement and resulted to diplopia24. In addition to donor site scarring, poor cosmetics and probable infection are the disadvan taged of conjunctival autograft and mucous membrane grafts for ocular surface reconstruction23,24. Unlike these two techni ques, the method of AMT that we used in study did not develop complications of symblepharon and granulation tissue, although scar formation was noted in one case. This result supported our hypothesis that AMT has better cosmetic outcomes. Corneoscleral dellen, cicatricial reaction, epithelial inclusion cysts and graft edema may occur as a complication of conjunctival auto grafting, whereas AMT was associated with transient mild edema and hyperemia, which resolved with topical steroids and antibiotics25. In addition, AMT allows preservation of the conjunctiva for patients who might necessitate a glaucoma filtering procedure in the future26.
Currently, AM is used fresh or preserved; both were found to be equally effective, but fresh AM has the risk for transmission of infectious diseases. On the other hand, the preserved preparation comprises frozen and dehydrated options. Frozen AM is stored in a preserved medium, such as glycerol/DMEM; glycerol/Hanks' balanced salts; and dimethyl sulfoxide, then cryopreserved at -80 ºC. The AM is warmed to room temperature immediately before use. The dehydrated form of AM can be stored at room temperature for up to five years by applying low-energy electron beam radiation then preserving with low heat and air27. Cooke et al. compared the preserved forms with the fresh form of AM and concluded that cryopreservation retained the extracellular matrix of the AM and retained the activity of biologic factors, high molecular weight hyaluronic acid, heavy chain-HA complex, and pentraxin 3. However, dehydrated tissues were missing these biological components and were structurally compromised28. The superior benefit of the dehydrated form over frozen tissue was that it can be kept at room temperature and did not require cold chain transportation27. The anti-inflammatory and anti-angiogenic features of the AM make this biological membrane unique in fighting against inflammation, neovascularization, and pain29. The stroma of the AM suppresses the signal activity of transforming growth factor β and myofibroblast differentiation in the corneal, limbal, and conjunctival fibroblasts30. AMT facilitates epithelial healing and restores the entire ocular surface without formation of scar6. Because of this property, AM has been successfully used to reconstruct various ocular surface disorders, such as conjunctival neoplasms or scarring, chemical or thermal burns, advanced ocular cicatricial pemphigoid and Stevens-Johnson syndrome, partial or total limbal stem cell deficiency, recurrent pterygium, symblepharon, persistent epithelial defects, and corneal ulcers31-34. Studies that have been reported on these various disorders demonstrated that AM enabled epithelial healing and suppressed inflammation after surgical excision. These valuable features make the AM an ideal tissue for reconstruction in cases of extensive resection6-8. Espana et al. evaluated AMT after excision of large ocular surface neoplasms followed by adjunctive cryotherapy. They studied 16 eyes that had CIN, PAM, and malignant melanoma; after a follow-up period of 23 months, they found that ocular surface healing was rapid and complete in all cases. One complication of pyogenic granuloma was noted. Tumor recurrence occurred in 1 of 10 CIN cases, but none was observed in the patients with melanotic lesions35.
We also evaluated the long-term results of AMT for conjunctival surface reconstruction after removal of large conjunctival and limbal tumors and demonstrated satisfactory outcomes when combined with cryotherapy. In our biomicroscopic investigations, we observed that AM integrated into the recipient bed, behaved as a basement membrane, and achieved conjunctivalization. Minimal or no pain after surgery was a remarkable feature of AMT. When AMT was used, extensive areas of the cornea and conjunctiva were preserved without severe postoperative pain. This was presumably the mechanical effect of the membrane preventing exposure of the nerve endings36. In our study, 2 of the 15 patients who experienced significant postoperative pain were the same patients who had complications and recurrence. This result is noteworthy for surgeons who prefer to use AMT in such cases.
As a summary, the anti-scarring effects of AM prevented serious complications after extensive resection. We believed that AM could be useful for reconstruction because of its unique features as an ideal tissue for progression of stem cells in cases of partial limbal deficiency after excision of extensive ocular surface tumors. In comparison with other techniques, such as mucosal or conjunctival autografting, AMT might be a superior technique for ocular surface repair after removal of large limbal and conjunctival tumors. New studies that include a larger number of patients and longer follow-up period are required for further assessment of the risk of recurrence and other complications.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

