INTRODUCTION
Ocular allergies constitute a heterogeneous group of frequently recurring inflammatory diseases of the ocular surface, with manifestations that range from mild to severe. Allergic conjunctivitis encompasses seasonal conjunctivitis, perennial conjunctivitis, atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC), and giant papillary conjunctivitis1. AKC and VKC are the most severe forms because of the possibility of visual impairment caused by corneal scarring, irregular astigmatism, cataract, and glaucoma2,3. Glaucoma and cataract secondary to corticosteroid therapy are potential causes of blindness, which is a concern especially in young patients.
The treatment of ocular allergies involves a stepwise approach starting with preventative measures, such as avoidance of allergens, cold compress, and maintenance of a cool dry environment. Medical treatment, including preservative-free artificial tears, topical nonsteroidal anti-inflammatory drugs, and topical antihistamines or mast cell stabilizers are used for milder cases. In contrast, more severe cases are treated with a combination of antihistamines or mast cell stabilizers and topical or systemic corticosteroids, which is given either as an intensive short-term therapy or as a long-term treatment regimen4. The use of immunomodulators, such as cyclosporin A, is reserved for cases with steroid-dependent allergic keratoconjunctivitis or when the use of corticosteroids is contraindicated, for example in patients with advanced glaucoma5. In severe cases refractory to medical treatment, surgical treatment, including excision of the tarsal papillae, superficial keratectomy with or without amniotic membrane graft, and eyelid surgery, may be performed.
Topical tacrolimus (TCL) is a potent immunosuppressive drug that is widely used to prevent allograft rejection of transplanted organs, such as the liver and kidneys6. Its immunosuppressive effect is a result of the reduction in IL-2 production by T lymphocytes via a mechanism of action that is similar with that of cyclosporin. Moreover, previous studies reported that TCL as more potent and better tolerated than cyclosporine7,8. Moreover, topical TCL has been described to yield good results for AKC and other types of inflammatory diseases of the ocular surface9. The other therapeutic indications for which TCL gave satisfactory results were inflammatory diseases, such as Mooren's ulcer10,11, high-risk penetrating keratoplasty12, giant papillary conjunctivitis13, AKC14-16, VKC17, and anterior uveitis18. There are two concentrations of TCL ointment approved by the Food and Drug Administration (FDA) for the treatment of atopic dermatitis; these are the 0.1% preparation for use in patients over 16 years old and the 0.03% preparation for use in children over the age of 2 years. These reported benefits and few side effects of TCL, as well as the few available data in children, compelled us to carry out this work. The objective of the present study was to evaluate the use of topical TCL for the treatment of severe allergic keratoconjunctivitis in children.
METHODS
This prospective study evaluated the use of topical TCL in pa tients with severe ocular allergies. Patients who were on topical antiallergic treatment for severe allergic keratoconjunctivitis and who had suffered recent progression of signs and symptoms after discontinuation of topical corticosteroid therapy were included in the study. Allergic keratoconjunctivitis was defined as the presence of recurrent ocular surface inflammation characterized by keratitis, gelatinous limbal infiltration, and/or giant papillae on the tarsal conjunctiva. Patients under 7 years of age, those with diseases lea ding to scarring of the conjunctiva, those with infectious corneal ulcer, and those with a history of herpetic keratitis were excluded from the study. Patients who have undergone surgical excision of giant papillae on the tarsal conjunctiva and those who have used systemic immunosuppressors or supratarsal injection of corticosteroids within the previous 6 months were also excluded.
The use of a placebo control group in this trial was not allowed due to ethical considerations because it would have required some patients to receive nonuseful treatment. This design may have led to some bias in the estimation of treatment effect.
This study was undertaken at the Cornea and External Eye Disea se Clinic, Department of Ophthalmology, Federal University of São Paulo, Brazil from January 2009 to July 2011. It was approved by the institute's internal review board under reference #1912-10. All individuals who participated in the study and/or their legal guardians gave a written informed consent for the study.
The treatment protocol comprised application on both eyes of 0.03% TCL ointment (Ophthalmos, São Paulo, Brazil) twice daily for at least 1 year, olopatadine hydrochloride 0.2% (Patanol S®) once daily, and an ocular lubricant containing polyethylene glycol (Oftane®) four times daily. All patients had been using olopatadine for at least 1 month before the treatment regimen was started. Topical corticosteroid therapy was discontinued but was resumed for a few days and with fast tapering when exacerbations occurred and when the above-mentioned drugs were unable to control the allergic process.
At each visit, all patients underwent detailed ophthalmologic eva luation, including visual acuity, intraocular pressure measurement, and slit-lamp biomicroscopic examination. Dilated fundus examination was performed on the first visit and was repeated as needed. The eyes were photographed for later comparison following TCL use.
Biomicroscopy included evaluation of the following items, in accor dance with the score system shown in chart 1: papillary reaction, gelatinous limbal infiltrates, and corneal epitheliopathy. A score ranging from 0 to 9, with 9 being the highest and 0 being the lowest, was assigned to each eye prior to and following treatment to assess for any improvement in the patient's clinical status (Figure 1). Classification was made in accordance with chart 1.
Chart 1 Classification of the signs observed at biomicroscopy. A score of 0-9 was assigned to each eye
| Papillae |
| 0: Micropapillae of the upper tarsal conjuctival and/or conjuctival thickening |
| 1: Prominent papilary reaction in the upper/lower tarsal conjuctuva with thickening, hampering observation of the vascular pattern. |
| 2: Thickened conjuctival surface with many papillae (some giant) on the upper tarsal conjuctiva, preventing observation of the vascular pattern and/or fibrosis. |
| 3: Predominance of giant papillae on the upper tarsal conjuctiva. |
| Gelationous limbal infiltrates |
| 0: Normal limbus. |
| 1: Mild hyperemia an edema |
| 2: As above, together with the presence of Horner-Trantas dots in 1-2 quadrants. |
| 3: As above, together with the presence of Horner-Trantas dots in more than 2 quadrants. |
| Keratitis |
| 0: Absent |
| 1: Punctante epithelial |
| 2: Coarse puctante ephitelial keratitis and/or focal or diffuse or epithelial erosion |
| 3: Epithelial defect or shield ulcer |
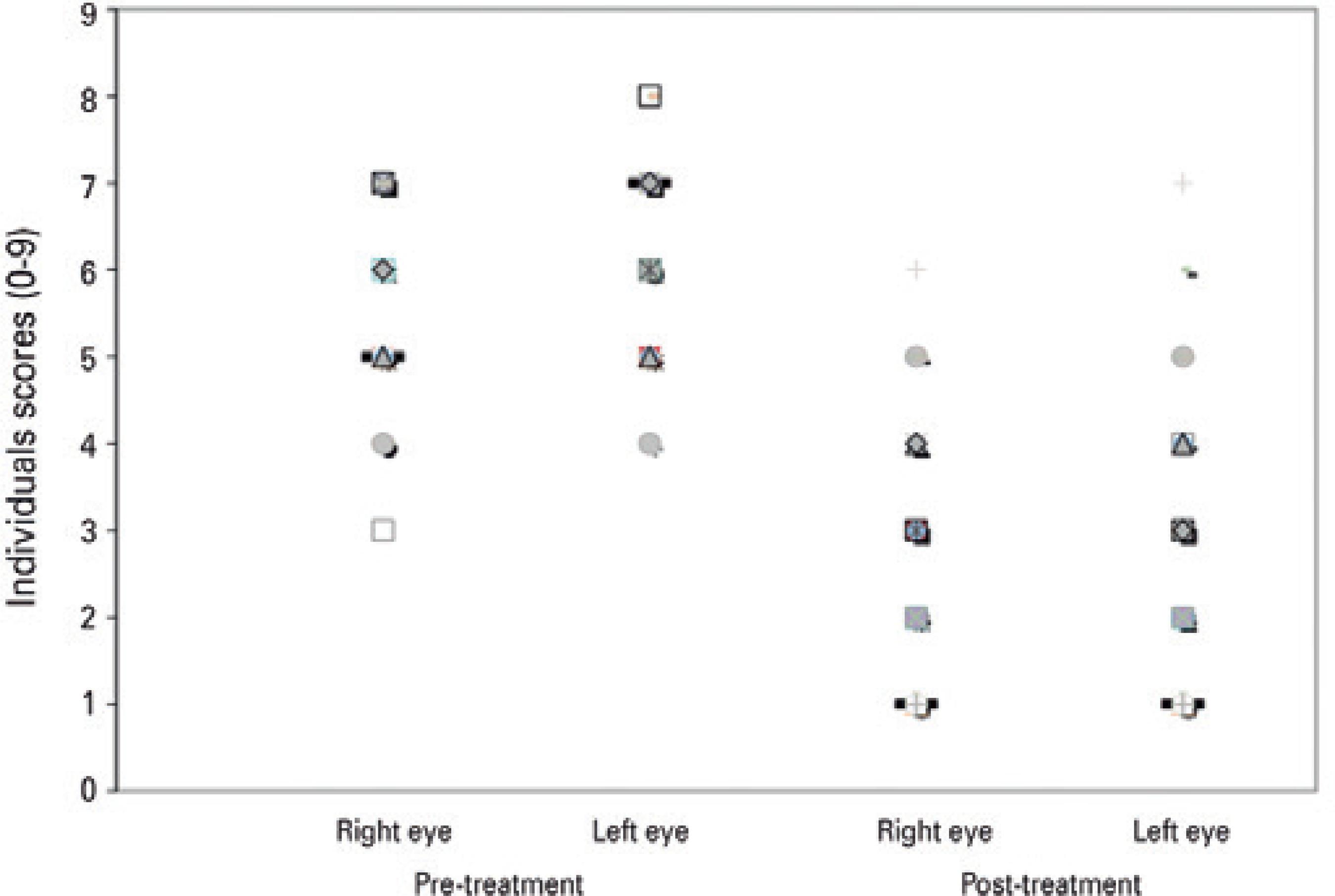
Figure 1 Clinical scores before and after tacrolimus ointment treatment for patients with allergic keratoconjunctivitis (n=66 eyes), mean follow-up duration was 13 months (range, 12-29 months).
The patients were followed up at scheduled visits on days 1 and 15 then monthly thereafter, except in cases that developed exacerbations occurred, in which case the patients were evaluated weekly. The final evaluation took place on July 2011.
Data were presented as frequencies and proportions or means and standard deviations. Wilcoxon test was used to compare the symptoms in the right and left eyes prior to and following TCL use. P-values <0.05 were considered statistically significant. The STATA software program, version 10 (College Station, Texas, USA), was used throughout the entire statistical analyzes. Data from each eye were analyzed separately to avoid the problem of rejecting useful data. Otherwise, the use of an overall summary of ocular findings for an individual may result in loss of relevant information and lead to less power and less precise estimates of effect, similar to when only one eye per individual is used.
RESULTS
The study included 33 patients, with a mean age of 12 ± 3.97 years (range, 7-16 years): 23 (69.7%) were men and 10 (30.3%) were women. The mean duration of keratoconjunctivitis symptoms was 5.8 ± 2.97 years (range, 1-15 years). VKC was diagnosed in 25 patients (75.76%), whereas AKC was seen in 8 patients (24.24%). Of the 25 patients with VKC, 5 (20%) had the limbal form, 9 (36%) had the palpebral form, and 11 (44%) had the mixed form. The associated diseases present prior to starting TCL treatment were rhinitis in 24 patients (72.73%), asthma in 14 (42.42%), dermatitis in 13 (39.39%), glaucoma in 6 (18.18%), and keratoconus in 4 (12.12%).
The mean follow-up duration was 13 months (range, 12-29 months). Of the 33 patients, 20 cases (60.61%) had successfully controlled disease and cessation of ocular allergy symptoms following TCL use, without recurrence of acute crisis (Figure 2). Recurrence was observed once in six patients (18.18%), twice in four patients (12.12%), and thrice in three patients (9.09%). As previously mentioned, short-course topical corticosteroids were administered as needed for some of these cases.
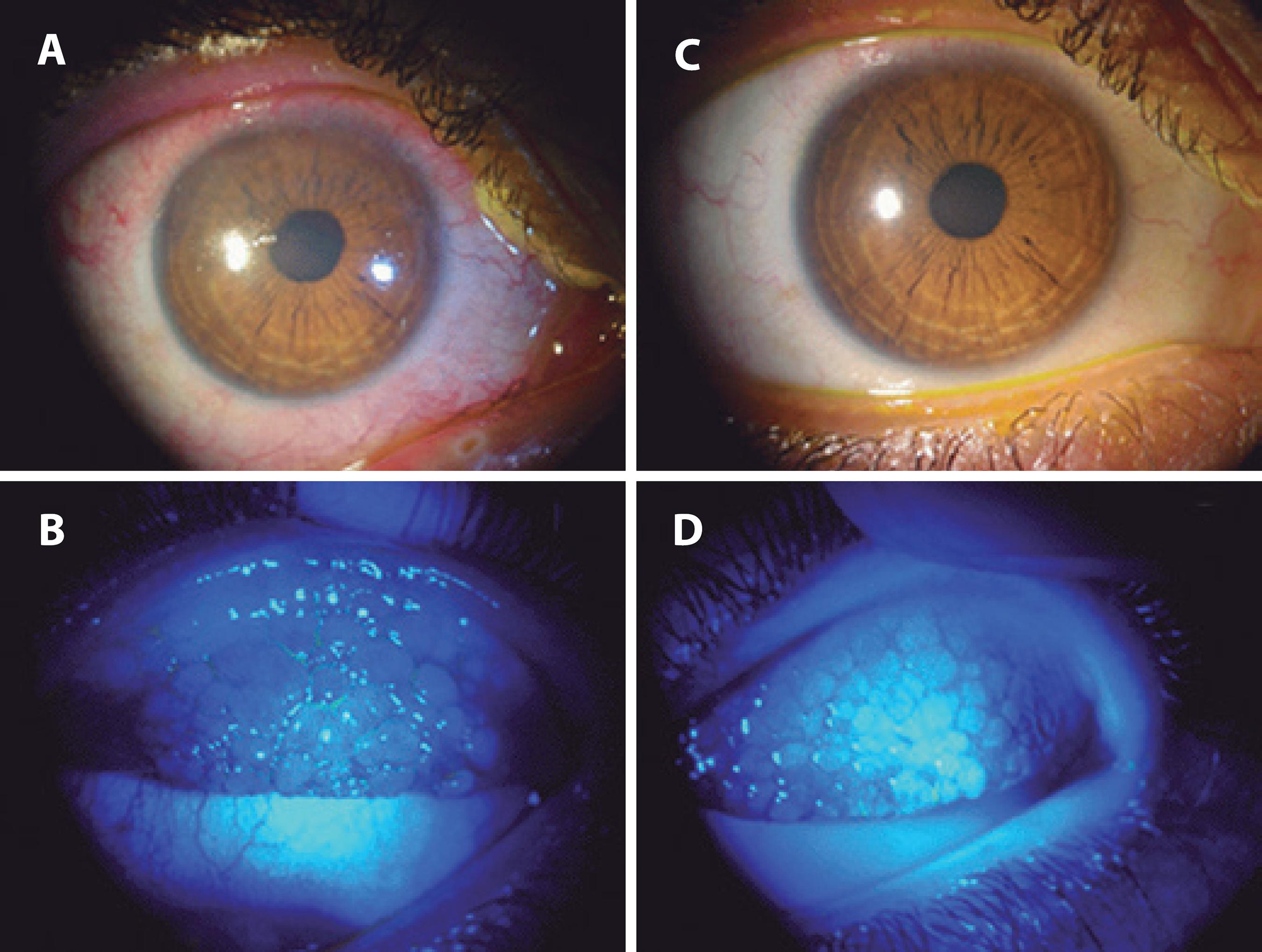
Figure 2 Pretreatment (A and B) and post treatment (C and D) with topical tacrolimus in a patient with vernal keratoconjunctivitis. Note the improvement in the papillae and keratitis after the treatment (C and D).
Four patients discontinued TCL because they developed irritation and burning sensation in the eyes. In one case, TCL was discontinued due to the appearance of ocular herpes (dendritic epithelial keratitis) following initiation of treatment; this patient was treated with topical antiviral medication for 10 days until complete resolution of the clinical condition. These five patients were excluded from the statistical analyzes.
Following TCL use, there were no reports of intraocular inflammation, glaucoma, increase of ocular pressure, and cataract.
DISCUSSION
The treatment of severe allergic keratoconjunctivitis involves cor ticosteroid eye drops in addition to topical antiallergic drugs and artificial tears. However, due to the possible side effects such as cataract and glaucoma, corticosteroid-sparing drugs are frequently needed4. Topical immunomodulators, such as TCL and cyclosporine, are safe alternatives for long-term treatment of these patients, with side effects that are generally transient and without rebound effects following discontinuation of the drug13.
The effect of topical cyclosporine depends on its concentration, which ranges from 0.05% to 2%; the higher the concentration, the poorer the tolerability and compliance with the treatment, particularly in young patients19-21. Daniell et al. showed that topical 0.05% cyclosporin failed to control VKC and AKC in steroid-dependent patients16. In another study conducted on patients with VKC, topical cyclosporin at 1% and 2% was proven to be effective for a long-term control of the allergy17. A systematic review suggested that topical cyclosporin may provide clinical and symptomatic relief from AKC and may help reduce topical steroid use in patients with steroid-dependent AKC22.
In the present study, TCL was chosen for the treatment of severe allergic keratoconjunctivitis because of the poor tolerability to cyclosporine,ambiguous results, and of little experience with TCL at the beginning of the clinical trial. Following the use of topical TCL 0.03% for severe cases of allergic keratoconjunctivitis in children, there were significant improvements in clinical signs of corneal epitheliopathy, limbal involvement, and papillary reaction; this reduced the need for topical corticoids and the associated side effects.
Labcharoenwongs et al. published a clinical trial comparing topical TCL and cyclosporine for VKC; twice daily use of TCL ophthalmic ointment tended to be more effective than cyclosporine in improving the ocular signs23. Systemic TCL, which is used to prevent organ rejection in individuals subjected to heart, lung, liver, kidney, pancreas, bowel, or bone marrow transplant, was reported to be 10-100 times more powerful than cyclosporine13. In an in vitro study, the immunosuppressive effect of TCL was found to be up to 100 times more potent than that of cyclosporine18. Topical TCL ointment and pimecrolimus cream have been commercially available for more than a decade and are the first and only drugs approved for chronic treatment of atopic dermatitis in pediatric patients because these are not associated with skin barrier compromise or increased percutaneous absorption. TCL at concentrations of 0.1% and 0.03% is currently available on the market for use in dermatology, with ex cellent results in atopic dermatitis24.
A study conducted on patients treated with topical TCL evalua ted cytology samples of the conjunctiva and found a statistically significant reduction in the number of eosinophils, neutrophils, and squamous metaplasia; no cases of cellular atypia were found25. According to Attas-Fox et al., the blood level of TCL following topical use is negligible26. Ohashi et al. reported that the use of topical TCL 0.1% for the treatment of allergic conjunctivitis was effective in improving the symptoms, in decreasing the size of the giant papillae on the tarsal conjunctiva, and in addressing corneal involvement27. Tzu et al. evaluated the long-term effectiveness of the combination of topical cyclosporine drops and TCL ointment in the treatment of steroid-dependent AKC; there was improvement of the symptoms and signs, and the number of flare-up episodes requiring topical steroids was low28.
The side effects of ocular use of TCL are generally tolerable, and only a few have reported the need to discontinue treatment9. There have been no reports on increase in intraocular pressure associated with the use of topical TCL; therefore, this drug appears to be a good option for patients with glaucoma or history of increased intraocular pressure following the use of topical corticosteroids, as well as for those with cataracts induced by corticosteroids29. Only 4 of the 33 patients in this study were unable to continue the use of TCL due to irritation and burning in their eyes. There are no data in literature describing the cause of TCL intolerance, as has been reported for cyclosporine19.
In patients given this immunosuppressive therapy with TCL, the risk for potential adverse effects, including reactivation of herpes and malignancy, should be taken into consideration. Development of cutaneous herpes simplex after application of topical TCL for atopic dermatitis was reported in one study30. In the present study, only one patient had an episode of ocular herpes after initiation TCL treatment. Although the use of topical calcineurin inhibitors, such as TCL, has been reported to have a theoretical risk for malignancy, including lymphoma, analyzes of epidemiologic and clinical data failed to demonstrate a causal relationship between TCI use and malignancy or lymphoma risk. Despite this potential risk, these drugs are considered safe and remain to be the only approved long-term treatment medication for children 2 years and older with atopic dermatitis31.
The present study suggested that topical TCL was effective and rendered satisfactory results, specifically significant improvements in the clinical signs of allergic keratoconjuctivitis, in children; it might be a new option for severe and challenging cases of ocular allergy. A longer follow-up time is required to evaluate the long-term reproducibility of these results. The absence of a control group for comparison with patients who received standard treatment for ocular allergy and the difference in treatment duration among patients may have led to some bias in the estimation of treatment effect.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

