INTRODUCTION
Fungal endophthalmitis is a rare condition, accounting for 8%-18% of culture-positive endophthalmitis(1) and often has a poor prog nosis. Given its low incidence, evidence-based data are unavailable(2). We report a case of endophthalmitis caused by an unusual yeast-like fungus, Stephanoascus ciferrii ( a teleomorph of Candida ciferrii) , that only responded to intravitreal caspofungin therapy.
CASE REPORT
In October 2014, a 57-year-old otherwise healthy woman presented to our clinic 2 weeks after an uneventful cataract surgery at another center. The best corrected visual acuity (BCVA) in her right eye was 0.2 on the logarithm of the minimum angle of resolution (logMAR) scale. Anterior pole examination revealed ciliary injection, 4+ anterior chamber cells, and a 1-mm hypopyon. Examination of the retina was possible (albeit difficult because of moderate anterior vitritis), and no white chorioretinal lesions or vitreous snowballs were visible. Anterior chamber paracentesis was performed (however, the subsequent culture was negative), and 0.1 mg of vancomycin was injected into the vitreous cavity (repeated at 48 h). We acknowledge that, according to the guidelines(3), a vitreous tap should have been performed and a second antibiotic, such as ceftazidime, should have been injected into the vitreous humor. The patient received subconjunctival injections of ceftazidime, intravenous ceftazidime, and oral moxifloxacin (based on our previous clinical experience, although we are aware that the use of systemic/periocular antibiotics is not supported by the guidelines(3)). However, the patient's visual acuity (VA) decreased over the next 10 days to 1.1 on the logMAR scale.
Subsequently, we performed a pars plana vitrectomy and posterior capsulotomy (Figure 1 A). Before opening the infusion line (we used 100 mg of vancomycin in 500 ml of balanced salt solution), we har vested a sample of vitreous gel that we immediately cultured on aerobic, anaerobic, and Sabouraud media. The outcome was favorable, and the patient was discharged from hospital after 5 days with a BCVA of 0 on the logMAR scale.
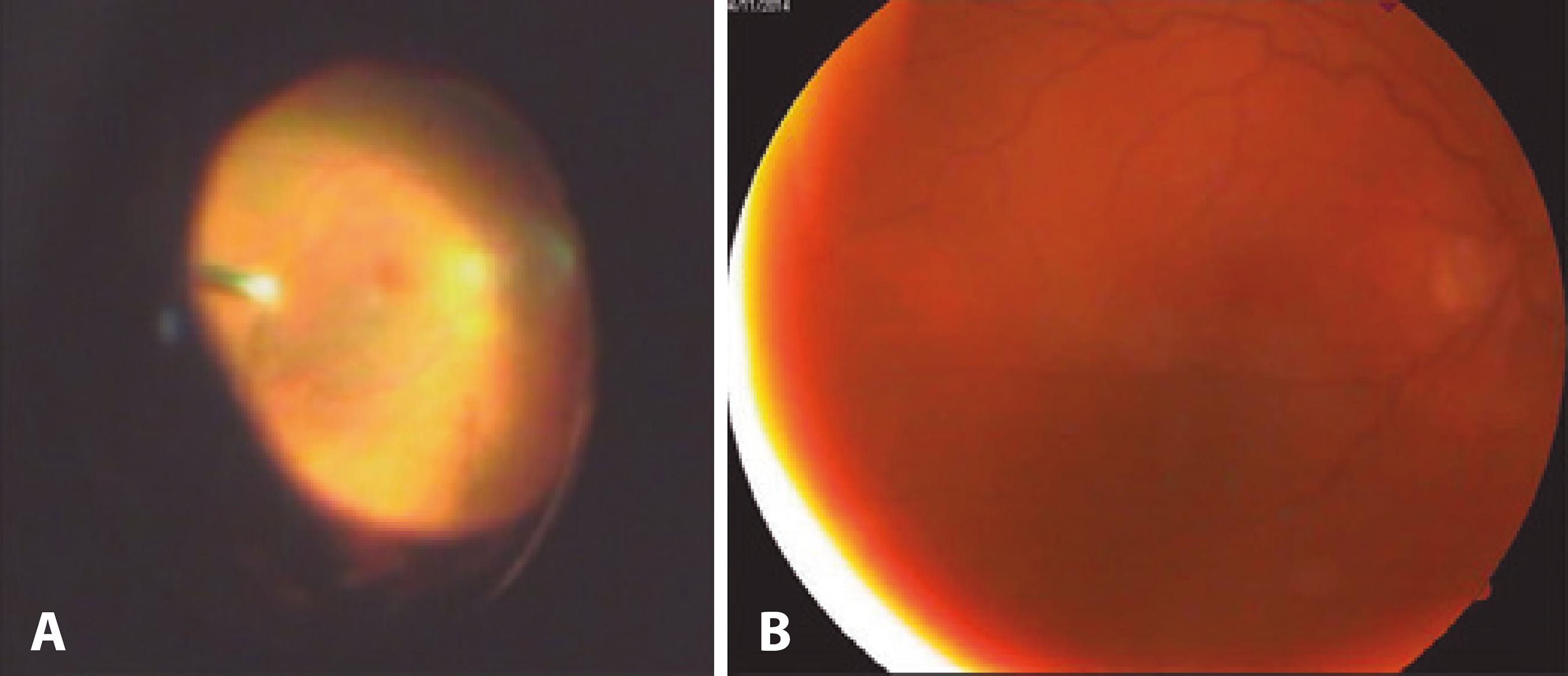
Figure 1 A) Image obtained during initial pars plana vitrectomy (rotated); B) Visualization of the fundus after intravitreal injections of 50 µg/0.1 ml caspofungin (6 weeks after the initial presentation).
Three weeks after her first admission, the patient returned with a VA of 1.6 on the logMAR scale, diffuse corneal edema, and 4+ anterior chamber cells. We performed explantation of the intraocular lens and capsular bag, and sent them for microbiologic examination. Because the clinical course was suggestive of fungal endophthalmitis, 0.1 mg of voriconazole was injected into the vitreous cavity. Parenteral antifungal therapy (200 mg of fluconazole daily) was initiated, and hepatic and renal functions were monitored during treatment. The patient consented to testing for human immunodeficiency virus (negative); her history and the findings of routine blood tests and an internal medicine exam failed to identify any cause of immune deficiency. The patient's VA slowly increased to 0.9 on the logMAR scale with aphakic correction, and she was discharged with instructions to continue oral fluconazole therapy.
Five weeks after her first admission, a mycologic exam conducted using standard laboratory identification procedures and a VITEK® 2C automated microbial identification system (bioMérieux, Marcy l'Etoile, France) came back positive for S. ciferrii. Antifungal susceptibility tes ting revealed that the fungus was resistant to fluconazole, voriconazole, and amphotericin B, but sensitive to caspofungin (minimum inhibitory concentration: 2 µg/ml). Concurrently, the patient had a counting fingers VA, diffuse corneal edema, and 4+ anterior chamber cells. Based on a literature search(1-8) we recommended intravitreal injections of caspofungin as a salvage therapy to the patient. She signed an informed consent form adapted to her situation.
Lavage of the anterior chamber and vitreal cavity was performed. Intravitreal injections of 50 µg/0.1 ml caspofungin were administered. Given the clinical response and lack of an alternative therapy, five intravitreal injections were administered at 48-h intervals (the total duration of the treatment was 8 days). The patient demonstrated a prompt response to this therapy, exhibiting a BCVA of 0.8 on the logMAR scale, minimal corneal edema, 1+ anterior chamber cells, and good visibility of the fundus (Figure 1 B). She was discharged, and minimal fluctuations in BCVA and clinical findings were recorded at monthly follow-up visits.
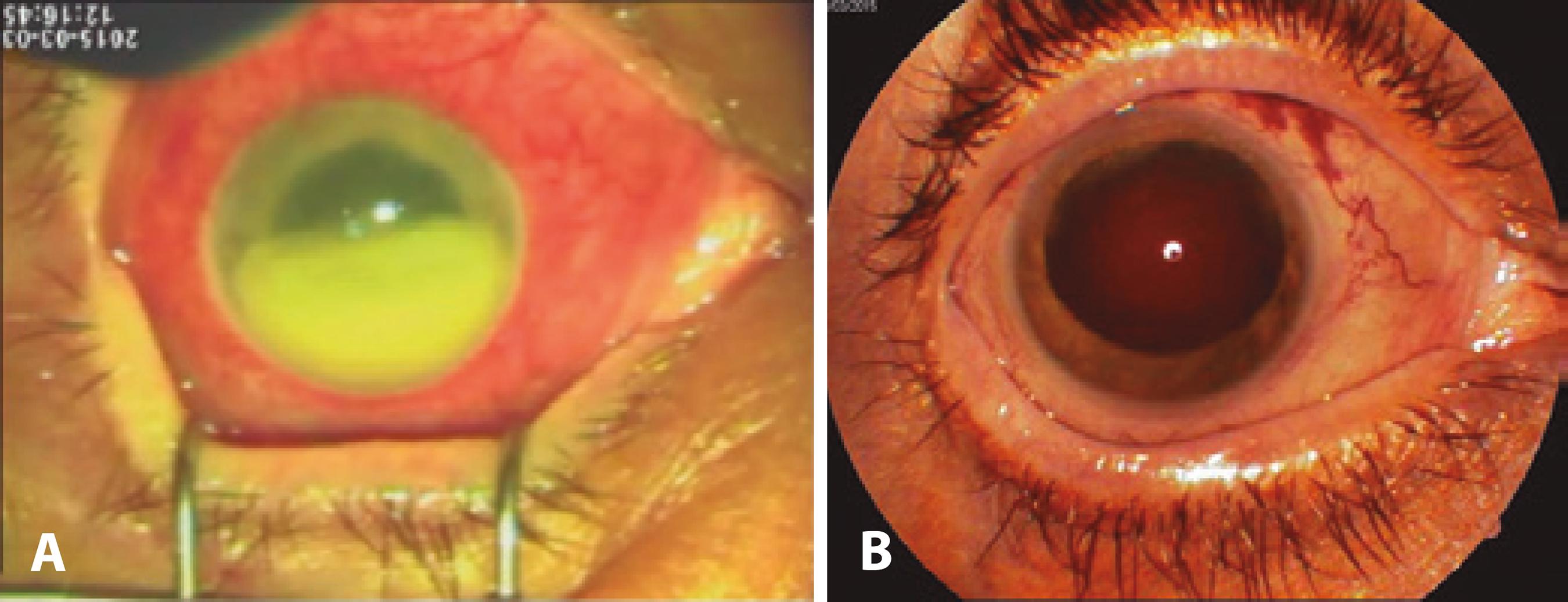
Between the fourth and fifth visits, the patient experienced a progressive recurrence of symptoms, and returned with a hand motion VA. She presented with corneal edema, and the inferior half of the anterior chamber was occupied by a dense, organized exudate (Figure 2 A). Considering the previous efficacy of caspofungin and lack of alternative treatment options, we repeated the intravitreal injections at a higher dose. After aspiration of the exudate in the anterior chamber and lavage of the vitreous cavity, five intravitreal injections of 250 µg/0.1 ml caspofungin were administered at 48-h intervals. The response to treatment was slow, but after 4 weeks, her BCVA had increased to 1.1 on the logMAR scale, with 1+ anterior chamber cells and fixed mydriasis (Figure 2 B). Further episodes of minimal cellular reactivity in the anterior chamber were treated with topical steroids, and the patient maintained the same BCVA (even after the occurrence of a secondary macular pucker) until 1 year of follow-up.
DISCUSSION
Although immunocompetent and lacking risk factors related to the initial cataract surgery, our patient presented with endophthalmitis caused by a yeast-like fungus. S. ciferrii (C. ciferrii) is an opportunistic pathogen that causes superficial infections in otherwise healthy in dividuals, but systemically invasive infections in immunocompromised patients(9,10). The first case of invasive infection in humans was reported in 2001 in a patient with acute leukemia who was already fluconazole-resistant(11). S. ciferrii strains have been described that are resistant to fluconazole, flucytosine, and itraconazole(9).
Caspofungin is an echinocandin antifungal drug that inhibits the enzyme β(1,3)-d-glucan-synthase, which is necessary for synthesis of the fungal cell wall(1). Because of its high protein-binding capacity and molecular mass, it is administered intravenously and exhibits minimal penetration into the eye. When caspofungin was administered intravenously in a rabbit model of uveitis (therefore with a disrupted blood-ocular barrier), it reached therapeutically relevant levels in the aqueous humor and cornea, but not the vitreous humor, of inflamed eyes(4). In a patient with Candida endophthalmitis, the disease pro gressed after monotherapy with intravenous caspofungin, and intraoperative vitreous sampling found that the concentration of caspofungin was undetectable(5). Systemic caspofungin was used in association with voriconazole for the treatment of three cases of endogenous fungal endophthalmitis(6).
In another case report, fungal endophthalmitis was successfully treated with intravenous caspofungin in a patient with candidemia associated with vitritis and a small retinal lesion in whom VA remained normal throughout 4 weeks of therapy(7). A response to intravenous caspofungin was also observed in 12 of 21 patients with endogenous fungal endophthalmitis included in controlled trials of echinocandins for the treatment of candidemia and invasive candidiasis, although detailed clinical information on these patients is unavailable(8).
In a study on the pharmacokinetics and safety of intravitreal injection of 50 µg/0.1 ml caspofungin, the concentrations of the drug in the vitreous humor at 1 and 16 h after injection were 6.06 and 2.04 µg/ml, respectively. After injecting up to 300 µg (corresponding to concentrations of up to 200 µg/ml in the 1.4-ml volume of the rabbit vitreous humor), no statistical differences were evident in a- and b-wave responses on scotopic electroretinography compared with control eyes(1).
In our patient, repeated intravitreal injections of 50 µg/0.1 ml caspofungin elicited a good clinical response, but the disease recurred after several months. Therefore, five injections of 250 µg/0.1 ml caspofungin were administered, and long-term remission of the infectious process and ambulatory vision were obtained. It is important to note that the injections were administered in a vitrectomized eye; thus, the clearance rate of the injected drugs was much faster.
The manufacturer of caspofungin recommends reconstitution of a 50 mg vial to a solution of 5 mg/ml followed by further dilution to 0.5 mg/ml (suitable for intravenous use and corresponding to 50 µg/0.1 ml). For the higher intravitreal dose, we diluted the reconstituted solution of 5 mg/ml to 2.5 mg/ml (corresponding to 250 µg/0.1 ml and still lower than the doses used in the safety study).
We were unable to distinguish the relative contributions of possible toxic effects and effects of the disease to the occurrence of sequelae (fixed mydriasis, low vision, and macular pucker formation), and we did not perform electroretinography in our patient.
To our knowledge, this is the first report of S. ciferrii endophthalmitis and its successful treatment using intravitreal caspofungin therapy. This case report provides clinicians with an alternative method of treating eyes unresponsive to conventional antifungal therapies.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

