INTRODUCTION
Ocular allergies constitute a heterogeneous group of frequently recurrent inflammatory diseases of the ocular surface, ranging from mild to severe manifestations. Allergic conjunctivitis encompasses seasonal conjunctivitis, perennial conjunctivitis, atopic keratoconjunctivitis (AKC), vernal keratoconjunctivitis (VKC), and giant papillary conjunctivitis(1). AKC and VKC are the most severe forms due to potential visual impairment caused by corneal scarring, irregular astigmatism, and keratoconus(2-3). Although glaucoma and cataract secondary to corticotherapy also represent potential causes of blindness, some patients show such severe clinical presentation and recurrence that the use of glucocorticosteroids is required to control the disease.
The treatment of ocular allergies involves the use of preventive measures such as avoiding exposure to specific and nonspecific agents that trigger the allergy, and the use of topical and/or systemic drugs. Milder cases can be treated with cold compresses, preservative-free artificial tears, topical nonsteroidal anti-inflammatory drugs, and topical antihistamines/mast cell stabilizers. More severe cases are treated with an association of antihistamines/mast cell stabilizers and topical or systemic corticosteroids, either as intensive short-term therapy or as long-term treatment regimes. The use of immunomodulators such as cyclosporine A and tacrolimus is reserved for cases of steroid-dependent allergic keratoconjunctivitis due to the known side effects of long-term use of steroids, or when the use of corticosteroids is contraindicated. Surgical treatment such as resection of the giant papillae may be used in severe cases in which there is persistent corneal injury(4,5).
Some patients demonstrate difficult disease control even when on systemic immunosuppressors, perhaps due to discontinuation or low adherence to the treatment, considering its high frequency of administration and/or high cost.
Successful management of refractory VKC with supratarsal injection of corticosteroids has been reported(3,6-9). The current study tested supratarsal injection of triamcinolone acetonide as a treatment option in difficult cases of recalcitrant VKC in children.
METHODS
This prospective study evaluated the supratarsal injection of 20 mg triamcinolone acetonide in children with severe VKC, defined as the presence of recurrent ocular surface inflammation characterized by diffuse punctate keratitis or repetitive shield ulcers, gelatinous limbal infiltration, and/or giant papillae on the tarsal conjunctiva.
The sample consisted of children who were not responding or inadequately responding to topical therapy with topical antiallergic treatment (olopatadine 0.2% once per day or 0.1% twice daily, or epinastine 0.05% twice daily) and preservative-free tear substitutes, and who had suffered recent deterioration in signs and symptoms following discontinuation of topical corticosteroid therapy (0.1% dexamethasone or 1% prednisolone 4 times daily with gradual reduction of 1 drop every 5 days).
Patients under 7 years of age; those with diseases leading to scarring of the conjunctiva, infectious corneal ulcer, and history of herpetic keratitis; those who had been submitted to surgical excision of giant papillae on the tarsal conjunctiva; and those who wear contact lens were excluded from the study.
This study was designed at the Cornea and External Eye Disease Sector, Department of Ophthalmology, Federal University of São Paulo, Brazil, from January 2009 to December 2014, and was approved by the institute's Internal Review Board (reference #0357/09). All participants and/or their legal guardians received information on the study and signed informed consent forms.
After applying topical anesthesia using 1% tetracaine hydrochloride, the upper lid was gently everted. Using a 25-gauge needle, 0.25 mL of lidocaine 2% was injected into the supratarsal space between the conjunctiva and Muller's muscle, approximately 1 mm above the superior tarsal border (Figure 1). Following the anesthesia, 20 mg triamcinolone acetonide was injected in the same potential space.
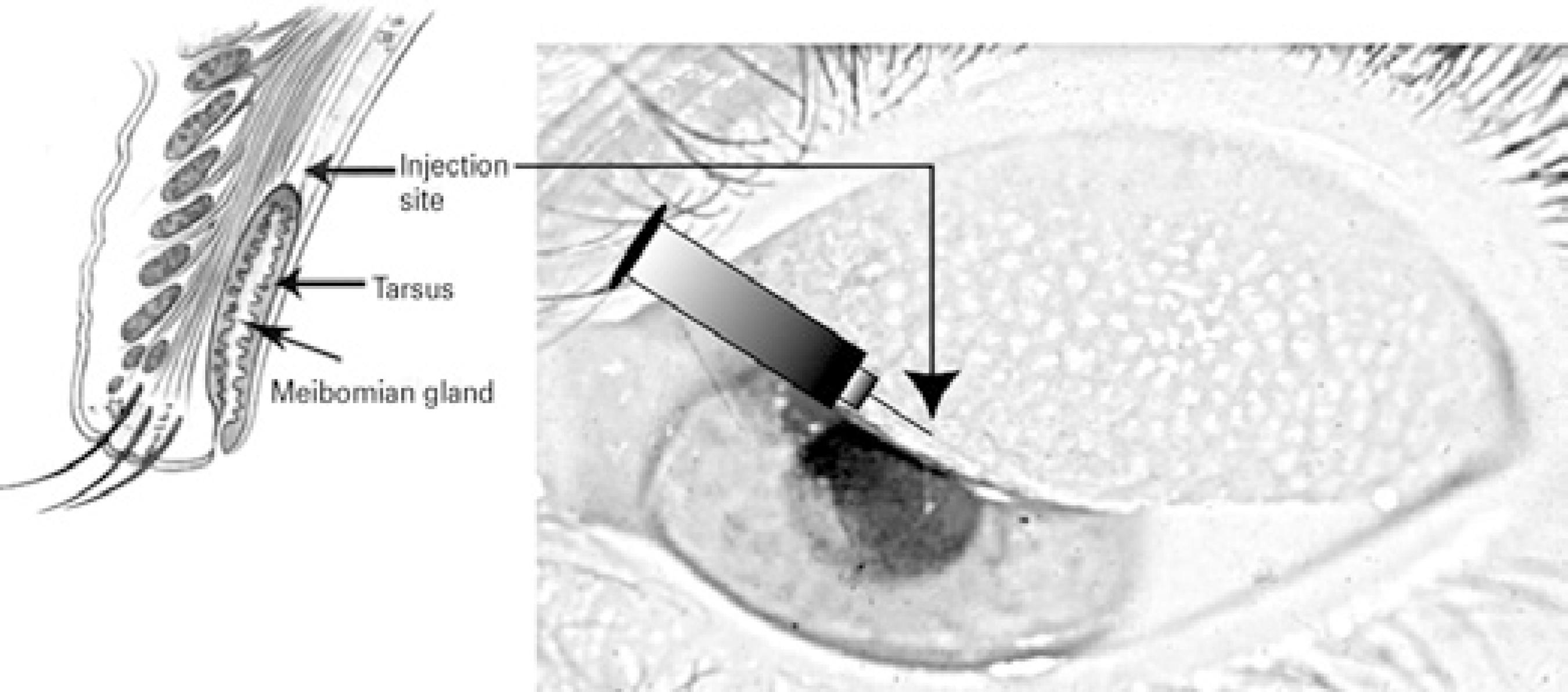
Figure 1 Injection site approximately 0.5-1 mm above the superior tarsal border in potential space between conjunctiva and Muller's muscle.
Patients received injections only in the eye(s) that showed recurring inflammation when topical corticosteroids were discontinued. After the application, each patient continued with the topical treatment that was already being used (e.g., olopatadine hydro chloride 0.2%, epinastine hydrochloride 0.05%, tacrolimus 0.03%, and carboxymethyl cellulose 0.5%). Topical corticosteroids were discontinued after injection.
Patients were evaluated at intervals of 1 day, 1 week, 2 weeks, and 1 month; they were then followed up monthly until 6 months after the injection, or whenever necessary (e.g .; when a new crisis arose). Treatment success was defined as a general reduction of symptoms and signs; for example, reduced cobblestone papillae size, gelatinous limbal infiltrates, and keratitis; clear conjunctiva; and decrease in Horner-Trantas dots and inflammation (lid edema, chemosis, pannus, and hyperemia). Disease recurrence was determined if symptoms and signs increased and attained or exceeded pre-treatment levels according to the patient's records and referral; in this case, reinjections were an option.
At each visit, patients underwent a detailed ophthalmic evaluation, including visual acuity, intraocular pressure (IOP) measurement, and slit-lamp biomicroscopy examination. Dilated fundus examination was performed at first visit and repeated as needed. The eyes were photographed for later comparison. Side effects following the steroid injection were closely observed; these included raised IOP, ptosis, infections, conjunctival scarring, and motility disturbance.
RESULTS
Twenty-seven injections were performed in 23 eyes of the 17 patients with severe VKC included in this study. Of these patients, 14 (82.3%) were male and 3 (17.7%) female. Mean patient age was 12.3 (SD=3.65; range: 7-19 years). Age of onset of symptoms associated with keratoconjunctivitis was 5.9 years (SD=3.29; range: 1-13 years). All patients were diagnosed with VKC: 7 patients (41.2%) with the palpebral form and 10 (58.8) with the mixed form (palpebral and limbal). Almost all patients (94.1%) had associated atopy, of which 16 patients (94.1%) had rhinitis, 5 (29.4%) had asthma, 4 (23.5%) had bronchitis, and 8 (47.0%) had dermatitis. The main signs observed were as follows: keratitis (100%), cobblestone papillae (88.2%), Trantas dots (52.9%), gelatinous limbal infiltrates (47%), and shield ulcer (47%) (Table 1).
Table 1 Demographic data and clinical features of patients (n=17)
| VKC patients (n) | Mean ± SD | |
|---|---|---|
| Age of injection (years) | 7-19 | 12.3 ± 3.65 |
| Age of onset | 1-13 | 5.9 ± 3.29 |
| Gender | % | |
| Male | 14 | 82.3 |
| Female | 3 | 17.7 |
| Associated atopy | 16 | 94.1 |
| Rhinitis | 16 | 94.1 |
| Asthma | 5 | 29.4 |
| Bronchitis | 4 | 23.5 |
| Dermatitis | 8 | 47.0 |
| VKC clinical presentation | ||
| Palpebral | 7 | 41.2 |
| Mixed | 10 | 58.8 |
| Signs involved | ||
| Cobblestone papillae | 15 | 88.2 |
| Gelatinous limbal infiltrates | 8 | 47.0 |
| Keratitis | 17 | 100.0 |
| Shield Ulcer | 8 | 47.0 |
| Tantras dots | 9 | 52.9 |
The mean follow-up time was 39.3 months (SD=19.21). Of the 17 patients, acute crisis was successfully controlled with remission of signs and symptoms in all (100%) for an average of 3.6 months (range: 1-16; SD=3.87). Complete resolution of lid edema and conjunctival chemosis, significant decline of keratitis and pannus, and reduction of giant papillae size with marked applanation were demonstrated in all patients (Figure 2). However, in 6 patients (35%), the disease was controlled for only one month following injection, 2 had severe recurrence of the ocular allergy and received systemic immunosuppression to control the disease, and 2 had repeated shield ulcers and underwent surgical resection of the giant papillae.
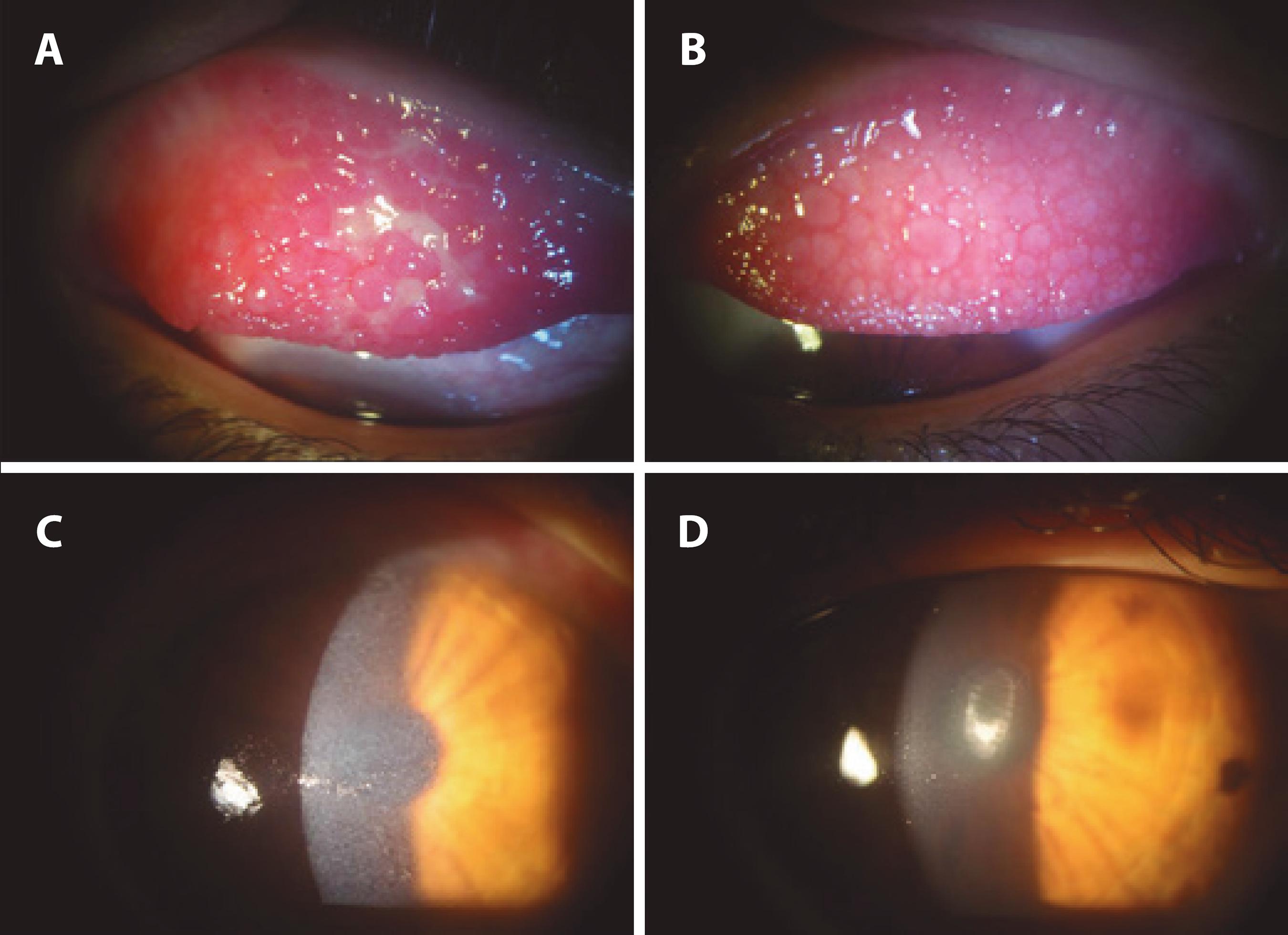
Figure 2 Efficacy of supratarsal triamcinolone injection after 1 week on the upper tarsal conjunctiva and cornea. A) Inflammation of the upper tarsal conjunctiva showing the Maxwell Lyon's sign and inflamed giant papillae. B) Improvement of conjunctival congestion, flattened giant papillae, mucous discharge, and edema after treatment. C) Diffuse keratitis was noted before treatment. D) Clear cornea with no keratitis after treatment.
Of the 17 patients, re-injection of triamcinolone due to worsening of the allergy was required in 6 (35%), 1 refused, and the other 5 were re-injected only in the affected eye; mean time between the first and second injection was 13.1 months (range: 2-38; SD=12.37). The remaining patients eventually experienced recurrences of the ocular allergy but in a milder form that could be controlled with topical drugs. No patients experienced complete disease remission. One patient showed increasing IOP, which rose from 11 to 16 mmHg after 4 days of the triamcinolone injection (Table 2).
Table 2 Results of supratarsal triamcinolone acetonide injection in vernal keratoconjunctivitis in children (n=17)
| VKC patients | % | |
|---|---|---|
| Signs and symptoms improvement | 17 | 100.0 |
| Early recurrence (1 month) | 6 | 35.0 |
| Need of reinjection | 6 | 35.0 |
| Months after 1st injection | Mean 13.1 (SD 11.3) | |
| Intraocular pressure elevation | 1 | 5.8 |
DISCUSSION
The treatment of patients with severe VKC involves-in addition to topical antiallergic drugs and artificial tears-the use of corticosteroid eye drops with their accompanying side effects such as cataract and glaucoma(4).
Treatment of such cases remains a challenge, and the supratarsal injection of triamcinolone acetate is an interesting therapeutic modality that shows rapid response and improvement with few side effects, and may lead to avoidance of systemic immunosuppression.
In the present study, a significant improvement was found with respect to keratitis, limbal involvement, and the appearance of the papillae following the supratarsal injection of 20 mg triamcinolone acetonide in severe cases of VKC in children, thus reducing the need for topical corticosteroids and their associated side effects. The injection indicated a more consistent result than did topical steroids alone, with more permanent control of the disease, or at least reduced intensity of the symptoms to be controlled henceforth with recurrent crisis. We recognize that VKC is a bilateral yet asymmetric disease, which is why the injection of triamcinolone was considered separately for each eye.
The rapid, initial symptomatic relief from supratarsal injection of triamcinolone acetonide is the result of local reduction of inflammation(8). Acetonide of triamcinolone has the capacity to inhibit intercellular adhesion molecule (ICAM-1), tumor necrosis factor, interleukins 1, 2, 6, and 8, and T cell-mediated cytotoxicity(7) factors that have been previously insinuated in the pathogenesis of VKC(10).
This treatment is also indicated for patients with problems of compliance to topical treatment due to high medication costs, difficulties in administering the drops to children, or a lack of responsibility or understanding by the caregiver.
Topical immunomodulators such as tacrolimus and cyclosporine A also represent options for long-term treatment of these patients, showing side effects that are generally transient and with no rebound effect following discontinuation of the drug(11). However, these drugs are very expensive in Brazil, and in some cases, recurrent allergy crises occur even while using them. Hence, when the patient is unable to use immunomodulators due to intolerable side effects or high cost, supratarsal injection can be a suitable option.
The main concern regarding the use of triamcinolone for the treatment of VKC is its influence on IOP. However, only one patient with VKC experienced elevation in IOP (≥5 mmHg) in our study, and other studies have shown similar rates of this complication(7,9). Other complications of the supratarsal triamcinolone injection have been reported(9), including blepharoptosis, skin depigmentation, infections, motility disturbance, and conjunctival scarring; none of these complications were observed in our patients.
The major difficulty encountered herein was convincing the children to cooperate with the procedure. In some cases, the child would not allow the injection in the second eye or refused a repeated injection a few months later (when applicable). Proper care should be taken to hold the patient's hand tightly in order to avoid accidents with the needle. Uncooperative children can be put under sedation to safely perform the procedure.
In conclusion, the treatment of severe acute VKC in children with supratarsal injection of 20 mg triamcinolone acetonide showed satisfactory results and was well tolerated by patients, suggesting that this treatment may constitute a safe option for severe and challenging cases. A significant improvement was found in ocular allergy symptoms and signs, with a reduction in the frequency of acute recurrences, albeit not leading to full disease remission. Similar results were reported in other studies(3,6-9).
Further studies with a larger number of patients and a control group are warranted to better assess the efficacy and safety of supratarsal injections of different doses of triamcinolone acetonide in the management of VKC and other types of ocular allergies.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

