INTRODUCTION
Ocular surface squamous neoplasia (OSSN) is used to classify a broad spectrum of dysplastic changes involving the epithelia of the conjunctiva, cornea, and limbus(1). The histological subtypes that com pound OSSN are squamous dysplasia, conjunctival/corneal intraepithelial neoplasia, and squamous cell carcinoma (SCC).
OSSN is a rare disease, with an incidence that ranges geogra phically (0.02 to 3.5 per 100,000); it is relatively common in Australia and African/tropical countries(2,3). In the last few decades, an increase in the incidence of OSSN has been observed in these countries, and this is mainly associated with sun exposure, human immunodeficiency virus (HIV), and human papillomavirus infection(4). There is no data on the incidence of OSSN in Brazil; however, although it is considered a rare disease in Brazil, it is still an important cause of ocular and systemic morbidity(1).
Currently, there are many treatment options for the management of OSSN, and selecting the most appropriate regime for a specific patient can minimize treatment-related morbidity and improve di sease control(5-12).
Historically, excisional biopsy with wide margins achieving clear margins was considered the gold standard of primary treatment. More recently, Shields and colleagues reported a method involving excisional biopsy with a no-touch technique followed by cryotherapy. This surgical technique has resulted in a low rate of recurrence in many institutions (7%-20%)(13).
Recent surveys have indicated that clinical practice patterns are evolving toward the use of topical chemotherapy drugs such as 5-fluorouracil (5-FU), mitomycin C (MMC), and interferon alfa-2b (IFNα2b) as monotherapy alone(14). Topical monotherapy has an advantage over surgery of treating the entire subclinical disease site. However, unlike surgery, tumor resolution is not immediate. Therefore, there is currently controversy regarding the best primary treatment option for OSSN(14). The intention of the present systematic review is to analyze the level and grade of recommendation of the evidence to guide ophthalmologists in the clinical treatment of OSSN.
METHODS
Literature search and selection criteria
The MEDLINE and Cochrane databases were searched using variations of the terms "conjunctival intraepithelial neoplasia," "ocular surface squamous neoplasia," "interferon-α2b," "5-fluorouracil," and "mitomycin C." All abstracts, studies, and citations found by these searches were reviewed. The information from each study was extracted independently by two reviewers.
Studies included
Studies were eligible if they were a randomized clinical trial, or a prospective comparative or single-arm study evaluating the treatment outcomes of adjuvant treatment or sole topical chemotherapy treatment for OSSN. Retrospective studies comparing different techni ques with a sample larger than 20 patients and at least 2 years of follow-up were permitted in this review.
Patients
This systematic review only included studies on OSSN patients (with SCC T1-2/N0, according to (Table 1) treated by surgical resection followed by adjuvant treatment or OSSN patients (with SCC T1-2/N0) treated by primary topical chemotherapy using the following drugs: MMC, 5-FU, or IFNα2b (Table 2).
Table 1 AJCC tumor stage for squamous cell carcinoma
| Tumor |
| Tx: Primary tumor cannot be assessed |
| T0: Tumor absent |
| T (is): Tumor present as carcinoma in situ/CIN |
| T1: Tumor present with largest basal diameter <5 mm |
| T2: Tumor present with largest basal diameter >5 mm, without invasion of adjacent structures |
| T3: Tumor invades adjacent structures excluding the orbit |
| T4: Tumor invades orbit with or without further extension |
| T4a: Tumor invades orbital soft tissues, without bone invasion |
| T4b: Tumor invades bone |
| T4c: Tumor invades adjacent paranasal sinuses |
| T4d: Tumor invades brain |
| Regional lymph nodes |
| Nx: Regional lymph node cannot be assessed |
| N0a: No regional lymph node metastasis, biopsy done |
| N0b: No regional lymph node metastasis, no biopsy done |
| N1: Regional lymph node metastasis |
| Distant metastasis (M) |
| M0: No distant metastasis |
| M1: Distant metastasis |
Table 2 Drug classification, mechanism of action, and administration of topical chemotherapy included in the systematic review
| Drug | Drug classification | Mechanism of action | Administration | Side effects |
|---|---|---|---|---|
| MMC | Alkylating agent | Generates free radicals under aerobic conditions | Topical 0.02-0.04% | Conjunctival hyperemia |
| ↓ | 0.04% (0.4 mg/ml) | Blepharospasm | ||
| Cytotoxicity | 4 times a day | Corneal punctate erosion | ||
| ↓ | 4 days a week | Punctal stenosis | ||
| Lipid peroxidation | 4 weeks | Limbal stem cell deficiency | ||
| ↓ | Treatment-free interval of 2 weeks | |||
| →; Inhibition of DNA and protein synthesis | ||||
| → Inhibits cell migration and production of extracellular matrix | ||||
| 5-FU | Pyrimidine analog | Inhibits thymidylate synthetaseInhibits production and incorporation of thymidine into DNAInhibits RNA synthesis | Topical 1% 5-FU 1% is used 3-4 times a day continuously for 3-4 weeks or 3-4 times daily for 4-7 days with 30-45 days off and cycles repeated |
Eyelid erythema |
| Conjunctival hyperemia | ||||
| Corneal punctate erosion | ||||
| IFNα2b | Type 1 IFN | Immune-mediated suppression of IL-10, stimulates IL-2 and IFN-γ mRNA | Topical 1 million IU/ml four times daily or intralesional 3 million IU/ml 10 million IU/ml subconjunctival injections administered weekly | Superficial punctate keratopathy |
| Anti-proliferative | Follicular conjunctivitis | |||
| Anti-viral | Systemic flu-like syndrome | |||
| Fever/myalgia |
Intervention
The intervention was sole topical or adjuvant chemotherapy treatment including MMC, 5-FU, or IFNα2b with any administration regimen (Table 2).
Outcomes
The recurrence and overall complication rates were the primary outcomes evaluated. The level of evidence and grade of recommendation were categorized according to the guidelines of the Oxford Centre for Evidence-based Medicine.
Five different clinical scenarios were identified: (1) adjuvant chemotherapy compared with surgery excision; (2) primary chemotherapy versus surgical excision; (3) adjuvant chemotherapy versus primary topical chemotherapy; (4) adjuvant treatment after surgery with different chemotherapy drugs; and (5) primary chemotherapy with different drugs.
RESULTS
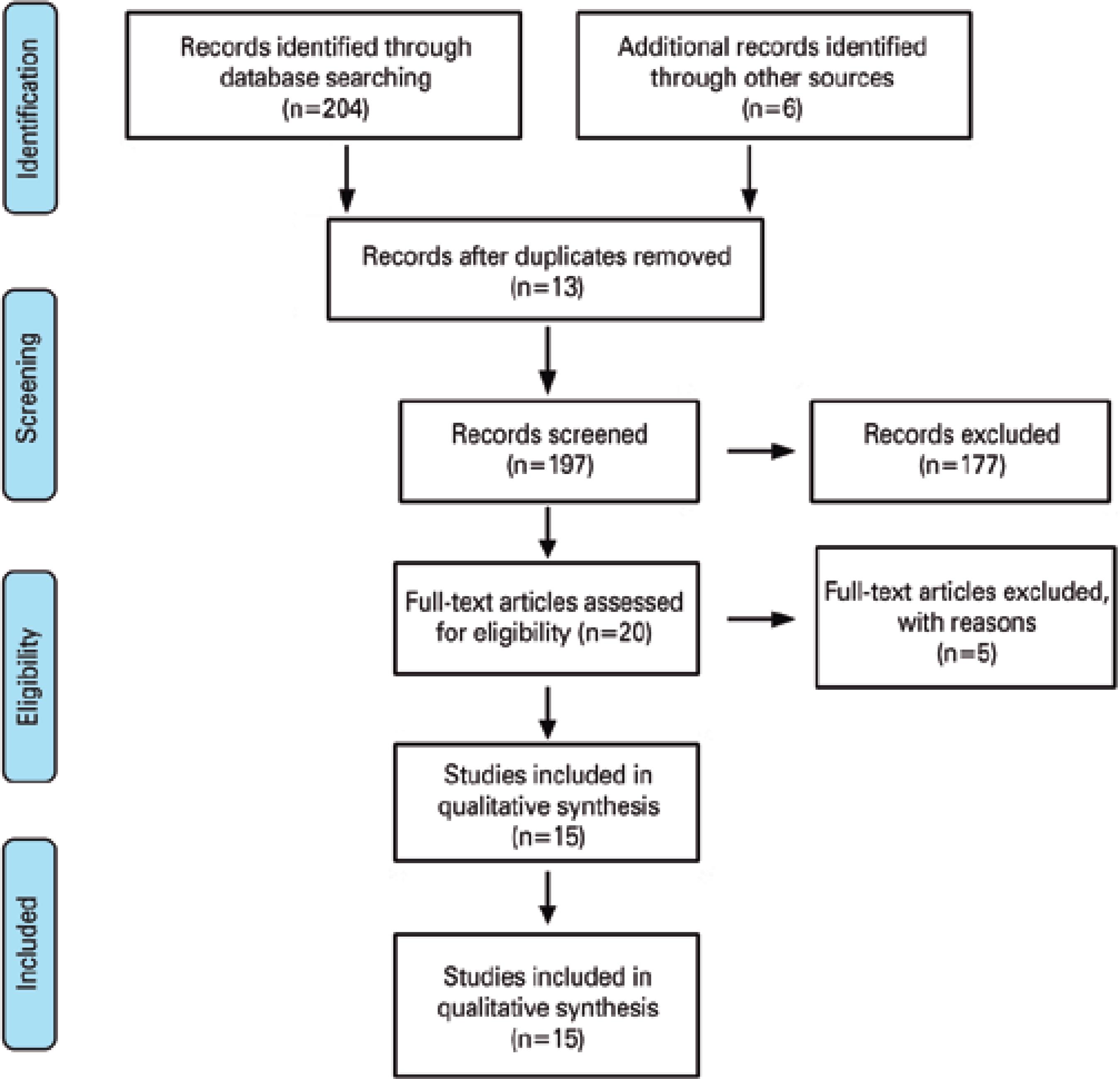
The searches resulted in 204 potential studies for the systematic review. After screening, only two randomized trials (146 patients), six prospective non-comparative studies (389 patients), and seven retrospective studies (442 patients) fulfilled the criteria to be included in this review (total patients=977). A flowchart of the included and excluded studies is shown in figure 1.
Adjuvant chemotherapy compared with surgery excision
The searches found one trial evaluating the role of adjuvant 5-FU after surgical resection for OSSN. In 2016, Gichuhi and colleagues published a randomized controlled trial of patients with OSSN(15). In this trial, after surgical resection, patients were randomized to receive topical 5-FU 1% or placebo four times a day for 4 weeks. They allocated 49 patients to receive adjuvant treatment with 5-FU and 49 to placebo. The recurrence rate was 11% in the 5-FU group and 36% in the placebo group (P=0.01). Side effects related to the treatment occurred more commonly in the 5-FU group, although they were transient and mild. Ocular discomfort (88%), epiphora (49%), and eyelid skin inflammation (15%) were the most common side effects in the 5-FU group (level of evidence Ib, grade of recommendation A).
We found six prospective non-comparative studies evaluating the treatment outcomes of adjuvant chemotherapy (three MMC and three 5-FU) after surgical excision(6,7,9,16-18). The recurrence rate after treatment ranged from 0% to 4.3% in the studies administering MMC 0.02%-0.04% (181 patients). In the studies with adjuvant 5-FU (185 patients), the recurrence rate ranged from 1% to 7.3%. All the side effects were graded as mild (5-FU=57%-69% and MMC=17.4%-41%) and resolved with medication discontinuation (Table 3) (level of evidence IIb, grade of recommendation B). Sturges et al. retrospectively described the long-term outcomes of 29 patients with OSSN treated with topical IFNα2b or surgical resection(19). Patients were treated with topical IFNα2b (15 patients) or resection with wide surgical margins (14 patients). Patients in the IFN group with non-complete response (failure) were crossed over to the surgery group. Only two failures in the IFN group subsequently underwent surgical excision. No patients in either group developed recurrence during the study period (median follow-up=35.6 months) (level III, grade of recommendation C). The outcomes of the other retrospective studies using MMC(20,21), 5-FU, and IFNα2b are summarized in table 4.
Table 3 Recurrence and side effects in prospective studies utilizing 5-FU and MMC for localized OSSN
| Author | Parrozani et al.(16) | Bahrami et al.(6) | Ballalai et al.(7) | Gupta et al.(17) | Bahrami et al.(6) | Chen et al.(9) | Rudkin et al.(18) |
|---|---|---|---|---|---|---|---|
| Type of study | Prospective, non-comparative case series | Prospective, non-comparative case series | Prospective, non-comparative case series | Prospective, non-comparative case series | Prospective, non-comparative case series | Prospective, non-comparative case series | Prospective, non-comparative case series |
| Regimen | 5-FU 1% QID for 4 weeks as primary or adjuvant therapy | 5-FU 1% QID for 2 weeks as adjuvant therapy | MMC 0.02% QID for 28 days | MMC 0.04% QID week on, week off for two or three cycles as adjuvant therapy | MMC 0.04% QID week on, week off for two or three cycles as adjuvant therapy | MMC 0.04% QID week on, week off for two or three cycles as adjuvant therapy | 5-FU 1% QID for 2 weeks as adjuvant therapy |
| Tumor type | Localized OSSN | Localized OSSN | Localized OSSN or SCC | Localized OSSN | Localized OSSN | Localized OSSN | Localized OSSN |
| Number of patients | 41 | 89 | 23 | 90 | 64 | 27 | 55 |
| Mean follow-up (months) | 90 | 37 | 46 | 56.8 | 61 | 27 | 23 |
| Recurrence rate | 7.3% primary | 1% | 4.3% | 0% adjuvant | 0 | 0 | 1.5% |
| 0 adjuvant | 12.5% primary | ||||||
| Rate of side effects | NR | 69% | 17.4% | 41% | NR | 57% |
Table 4 Recurrence rate and side effects from retrospective studies
| Author | Sample | Treatment | Recurrence | Side effects |
|---|---|---|---|---|
| Shields et al.(5) | 81 | IFNα2b alone (n=22) | 5% median=1 year | Conjunctival hyperemia: 4, 5% |
| IFNα2b + surgery (n=59) | Ocular irritation: 3, 4% | |||
| Superficial punctate keratitis: 3, 4% | ||||
| Conjunctival follicles: 1, 1% | ||||
| Flu-like syndrome for 1 day: 7, 9% | ||||
| Sturges et al.(19) | 29 | IFNα2b alone (n=15) Surgical excision (n=14) IFNα2b 1 million IU/ml, or 2 million U/ml, all 4 times per day, or 3 million IU/ml thrice daily If the patient failed to regress within 2 months of IFNα2b, they were crossed over to the surgery group |
IFNα2b alone=13.3% underwent surgery Surgical excision=0 During the study period of 35.6 months, no recurrence was detected |
NR |
| Kusumesh et al.(10) | 51 | IFNα2b alone (n=26) | Follow-up: 3.5 months in the IFNα2b group and 1.5 months in the MMC group | Adverse effects occurred in 3 (12%) patients using IFNα2b and 22 (88%) patients using MMC |
| 0.04% MMC alone (n=25) | ||||
| Author | Sample | Treatment=MMC | Recurrence | Side effects |
| Birkholz et al.(20) | 32 | Excision (n=15) Excision + MMC (n=17). Intraoperative MMC 0.02% or 0.04%. Postoperatively, MMC 0.02% was given TID for 2 weeks. Three cycles of 2 weeks on and 2 weeks off MMC as primary treatment or surgical adjuvant. MMC 0.04% four times a day for 3 weeks on, 3 weeks off, 3 weeks on, with topical steroid and lubricants throughout |
Excision alone=66.7% Excision + MMC=7.7% 31.7 months’ follow-up 67% recurrence rate with primary topical treatment after a mean follow-up of 50 months. All cases salvaged by surgical excision with no recurrence |
Discomfort in all patients |
| The most common complication was allergy. 64% the majority of complications developed after the second 3-week cycle. The most common long-term complication was continuing mild keratoconjunctivitis | ||||
| Author | Sample | Treatment=5-FU | Recurrence | Adverse effects |
| Russel et al.(21) | 58 | MMC as primary treatment or surgical adjuvant. MMC 0.04% four times a day for 3 weeks on, 3 weeks off, 3 weeks on, with topical steroid and lubricants throughout |
67% recurrence rate with primary topical treatment after a mean follow-up of 50 months. All cases salvaged by surgical excision with no recurrence | The most common complication was allergy. 64% the majority of complications developed after the second 3-week cycle. The most common long-term complication was continuing mild keratoconjunctivitis |
| Author | Sample | Treatment=5-FU | Recurrence | Adverse effects |
| Rudkin et al.(8) | 38 | 5-FU 1% QID × 2 weeks MMC 0.04% × 2–3 cycles Each cycle lasted 1 week, followed by 1 week drug off. Patients with no response had excision with a 2-mm margin + cryotherapy |
MMC=7 patients | 5-FU 1%: 58.3%, with a single case of focal paracentral corneal stromal melt. MMC 0.04%: resulted in transient drug-related complications in 59% of cases |
| 5-FU=3 patients |
Primary topical chemotherapy effectiveness
Hirst conducted the sole randomized clinical trial assessing the effectiveness of primary topical treatment with MMC in localized OSSN(22). Forty-eight consecutive patients with OSSN were included. One drop of MMC (0.4 mg/ml) or placebo was administered four times a day for 3 weeks, with crossover of the drops if there was no regression within 6 weeks. None of the patients receiving placebo achieved complete response, whereas 93% of the MMC group resulted in complete response. There were no severe side effects (level of evidence Ib, grade of recommendation A).
Adjuvant chemotherapy versus primary topical chemotherapy
The sole study comparing adjuvant treatment versus topical treatment was conducted by Shields et al.(5). In this study, adjuvant IFNα2b was retrospectively compared with primary IFNα2b therapy in 80 patients with OSSN. Primary treatment with IFNα2b alone resulted in complete response in 75% (3/4) Tis, 100% (8/8) T1, and 70% (7/10) T3, whereas IFNα2b plus surgery (n=59) resulted in 100% complete response independent of the tumour stage. Tumor recurrence was seen in 5% of cases treated with IFNα2b alone over a median follow-up of 1 year (Table 4) (level of evidence III, grade of recommendation C).
Adjuvant treatment after surgery with different chemotherapy drugs
Bahrami et al. retrospectively compared 89 eyes treated with adjuvant 5-5-FU and 64 eyes treated with adjuvant MMC(6). The surgical procedure consisted of excision ± cryotherapy followed by the administration of topical 5-FU 1% QID for 2 weeks or topical MMC 0.04% QID for 2-3-week cycles. The median follow-up was longer than 2 years in both groups, and there was one recurrence in the 5-FU group and no recurrences in the MMC group. Side effects occurred in 69% of the 5-FU patients and 41% of the MMC patients (Table 4) (level of evidence III, grade of recommendation C).
Primary chemotherapy with different drugs
We found two prospective non-comparative studies that evalua ted the treatment outcomes of primary topical chemotherapy (one MMC and one 5-FU). Balalai et al. evaluated the recurrence rate and long-term complications of MMC 0.02% for OSSN(7) in 23 patients. All patients achieved complete response after 1 month of treatment, and the rate of recurrence was 4.3% after 24 months of treatment. Corneal erosion developed in 17.4% of the patients. Parozzoni et al. prospectively evaluated 41 patients with OSSN who underwent primary topical treatment with 5-FU 1% QID for 4 weeks or adjuvant treatment(16). The rate of recurrence was 7.3% with 5-FU alone (level of evidence IIb, grade of recommendation B). Kusumesh et al.(10) included 50 patients with OSSN in a retrospective study treated with either topical IFNα2b (1 million IU/mL) or MMC (0.4 mg/mL), with 26 and 25 eyes receiving topical IFNα2b and MMC, respectively. Complete response was achieved in 89% and 92% with topical IFNα2b and MMC, respectively (P=0.67). The median time to lesion resolution was 3.5 months in the IFNα2b group and 1.5 months in the MMC group (P<0.005). Adverse effects occurred in 12% and 88% using IFNα2b and MMC, respectively (P<0.005) (level of evidence III, grade of recommendation C).
DISCUSSION
Over the past few decades, the standard treatment for OSSN has been surgical excision alone with a no-touch technique(13). However, a high rate of tumor recurrence has been reported with surgical resection of the neoplasia alone. The principal reason for the high recurrence rate with surgery alone is the microscopic extension of disease beyond the edge of the surgical margins. Currently, margins of at least 3 mm are considered to be safe and wide enough for OSSN(13). The main prognostic factors related to recurrence are the margin status, lesion size, and presence of pre- or invasive disease. For instance, Galor et al. evaluated the prognostic factors for recurrence in 389 excised OSSN lesions(23). The 1- and 5-year recurrence rates were 10% and 21%, respectively. In the univariate analysis, T2-3 lesions (P=0.07), the presence of positive margins (P=0.008), and higher grade lesions (carcinoma in situ and SCC versus dysplasia; P=0.02) were significant prognostic factors for recurrence, and adjuvant cryotherapy significantly decreased the risk of recurrence (P=0.03).
The purpose of the present systematic review is to analyze the evidence available in the literature to establish treatment recommendations for different clinical scenarios based on the level of evidence from the Oxford guidelines. For clinical scenario (1) (adjuvant chemotherapy compared with surgery excision), the best level of evidence to support adjuvant treatment after surgical excision came from a randomized clinical trial comparing topical 5-FU 1% with surgery alone. Note that all the patients in this study were African patients with T1-2/N0 OSSN, with disease restricted to one quadrant; further, the majority of these patients had HIV infection(15). Therefore, although this study provides a high level of evidence and recommendation, it has potential limitations that need to be considered. For us, the main limitation of this study was the higher recurrence rate achieved in the adjuvant and observation arms when compared with the rates reported by many other studies from countries with temperate climates. Multiple factors contributed to this difference, including the patients being infected with HIV, more aggressive tumors, and the short follow-up.
For clinical scenario (2) (primary topical chemotherapy effectiveness), the only randomized clinical trial investigating topical chemotherapy for OSSN was a crossover trial conducted by Hirst. This study showed that the topical chemotherapy group had a higher rate of complete response than the placebo group, which supports MMC 0.04% as a good therapeutic option(22).
However, for clinical scenarios (3)-(5) (adjuvant chemotherapy versus primary topical chemotherapy, adjuvant treatment after surgery with different chemotherapy drugs, and primary chemotherapy with different drugs, respectively), the evidence was not clear, which makes it difficult to come to a decision regarding recommendations of chemotherapy drugs or even the drug schedule in clinical practice for both adjuvant or topical treatment.
Looking at the treatment results from prospective non-comparative series, adjuvant MMC and 5-FU appear to produce similar effects in terms of disease control; however, MMC has a better toxicity profile Tables 3 and 4). Besides, the evidence for using IFNα2b is also of a low level and based on retrospective comparative studies or prospective single-arm studies(5,19). In these studies, the rates of recurrence and complete response were similar, and the toxicity profile of IFNα2b appears to be more favorable than that of MMC(24). Another point of difficulty in evaluating the literature was the absence of randomized or comparative studies assessing different drug dosages and schedules. There are no head-to-head studies comparing the same drugs with different concentrations or altered schedules. The lack of information on these factors affects the recommendations of the best treatment regimen regarding efficacy and safety. For instance, in a recent survey including 81 ophthalmologists, 79% of the responders considered that there was enough evidence for recommending topical monotherapy, but only 58% responded that they had ever prescribed topical agents as monotherapy(14). IFNα2b (56%) and MMC (37%) were the preferred first-line topical therapies, and MMC was the preferred adjuvant drug for post-surgical treatment. The survey detected a shift from surgical resection alone to excision followed by adjuvant treatment when comparing the results of the 2012 survey with those of the 2003 survey.
CONCLUSION
The results of this systematic review show that there is compelling evidence to resolve five important clinical scenarios regarding the use of adjuvant and primary topical chemotherapy treatment in OSSN. Although there are no head-to-head studies comparing different drugs or schedules for adjuvant and/or primary treatment, the results of prospective single-arm or retrospective studies show similar local control of the disease with MMC, 5-FU, and IFN. From the evidence available, IFNα2b appears to have the best toxicity profile, followed successively by MMC and 5-FU. However, because of the lack of trials investigating different dosages and schedules, it is difficult to affirm which drug has the better toxicity profile and which should be recommended in clinical practice. Even with these limitations, it is possible to make treatment recommendations to guide clinical practice (Table 5). Further studies are warranted to evaluate the use of different dosages, schedules, and concentrations in the treatment of OSSN.
Table 5 Treatment recommendations based on the level of evidence for OSSN
| OSSN ≤1 quadrant | • Excision biopsy + base edge cryotherapy + alcohol epitheliectomy is indicated (preferred). |
| • If post-op margins are negative, quarterly follow-up for 1 year is recommended to confirm absence of recurrence. | |
| • If post-op margins are positive, topical adjuvant chemotherapy (Table 2) should be considered, with monthly follow-up in the first 6 months and quarterly thereafter. If disease free for 1 year, 6 monthly follow-up is recommended. | |
| • Alternatively, diagnostic biopsy is recommended, followed by topical chemotherapy alone (Table 2) for dose, drug type, and schedule. | |
| • Alternatively, diagnostic biopsy is recommended, followed by topical chemotherapy alone (Table 2) for dose, drug type, and schedule. | |
| OSSN 1 ≤2 quadrants | • Diagnostic biopsy is required. |
| • Pre-invasive lesion: primary topical chemotherapy (Table 2) is indicated, with monthly follow-up in the first 6 months and quarterly thereafter. If there is recurrence or partial response, surgical excision is indicated. If there is no risk of producing limbal stem cell deficiency, excision biopsy + cryotherapy + topical chemotherapy is indicated if T2 or higher, or if positive margins are found. | |
| • Invasive lesion: pre-operative topical chemotherapy (Table 2) is indicated, followed by surgical excision + cryotherapy, with monthly follow-up in the first 6 months and quarterly thereafter. | |
| OSSN >2 quadrants | • Diagnostic biopsy is required. |
| • Pre-invasive lesion: primary topical chemotherapy (Table 2) is indicated, with monthly follow-up in the first 6 months and quarterly thereafter. If there is recurrence or partial response, surgical excision is indicated. | |
| • Invasive lesion: pre-operative topical chemotherapy (Table 2) is indicated, followed by surgical excision + cryotherapy, with monthly follow-up in the first 6 months and quarterly thereafter. If there is no response to chemotherapy, alternative treatment with brachytherapy or beta irradiation is recommended. |




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

