INTRODUCTION
Keratoconus is generally a progressive, bilateral cone-like ectasia of the cornea that affects approximately 1 in 2,000 people in the general population, which is usually diagnosed in young adult patients (1). It is characterized by localized corneal thinning and protrusion, which lead to high myopia and irregular astigmatism that reduces visual quality. The initial treatment of keratoconus is the use of glasses and/or contact lenses (1). More advanced stages of the disease are treated by various surgical procedures (2). However, the relief of keratoconus symptoms using currently available treatment modalities may not be permanent, and it remains impossible to predict with certainty how long favorable results will persist.
The pathophysiology of keratoconus remains poorly defined, but its etiology is known to be multifactorial and it is an autosomal dominant trait with variable phenotypic expression (2). It is associated with many ophthalmic and systemic conditions, such as Down syndrome, eye rubbing, mitral valve prolapse, collagen vascular disease, and a history of contact lens wear (2). Other conditions include eyelid diseases such as blepharoptosis (3) and floppy eyelid syndrome (4).
Keratoconus patients present with excessive eye blinking, and usually develop a routine of chronic eye rubbing with consequent excessive manipulation of the periocular area. Augmented pressure to the eyelids may cause further topographic changes to the cornea (5,6), and eyelid muscular force can translate to the ocular surface and exacerbate symptoms. It was therefore hypothesized that reducing eyelid muscular force may provide a novel treatment option for keratoconus that inhibited the progression of damage to the cornea.
The use of botulinum toxin type A (BTX-A) in medicine was first reported in 1980 for treating strabismus (7). It blocks the release of acetylcholine into the neuromuscular junction, leading to temporary muscle weakness or paralysis (8). BTX-A is now a front-line treatment option for focal dystonias such as essential blepharospasm and hemifacial spasm, and it has become a major therapeutic drug with valuable applications in diverse medical subspecialties (8). The purpose of this study was to evaluate, as a potential novel therapy for patients with keratoconus, whether reducing eyelid muscular force through the administration of BTX-A into the orbicularis oculi muscles could prevent the progression of corneal damage.
METHODS
Design
This parallel randomized clinical trial was conducted between September 2012 and October 2014 in the Department of Ophthalmology and Visual Sciences, Federal University of São Paulo, Brazil. The study was approved by the Institutional Ethics Committee and followed the tenets of the Declaration of Helsinki. All patients provided written informed consent. This manuscript has been registered (NCT01691651) and is publicly available at www.clinicaltrials.gov.
Participants
Patients were enrolled if they met the following inclusion criteria: documented keratoconus, best spectacle-corrected visual acuity (BSCVA) that could be measured by refraction, no major comorbidities such as diabetes, and aged 10-40 years. Exclusion criteria were having only one functional eye, previous ocular surgery, eyelid margin disease that did not respond to treatment (e.g., meibomian gland dysfunction and blepharitis), a known allergy to BTX-A or other medication components, a history of herpetic keratitis, concurrent corneal infection, pregnancy or nursing, and other ocular diseases that could affect visual acuity.
Sample size and randomization
The two main outcomes considered in this study were the dimensions of the palpebral fissure and keratometry measurements; the sample size was calculated on the basis of these. From a pilot study per formed in our department, we expected a mean ± standard deviation (SD) change in the height of the palpebral fissure of 2.0 ± 1.5 mm and an astigmatism change of 1.0 ± 0.8 diopters (D). We calculated that a sample size of 20 individuals/group would provide 90% power to detect these magnitudes of outcome differences in the present study.
Randomization was performed using a computer-generated randomization table (Stata v.11, Stata Corp, College Station, TX). The participants were allocated into either the control or the BTX-A injection group in a 1:1 ratio. Due to the design of this study (treatment versus no treatment), the examiner was not masked to the type of treatment during examination, although the statistician was masked during data analysis.
Measures
At the baseline and post-treatment follow-up examinations at 3, 6, 12, and 18 months, all participants underwent the following assessments: 1) uncorrected visual acuity (UCVA); 2) BSCVA; 3) manifest refraction in a bright environment; 4) slit-lamp biomicroscopy; 5) measurement of the palpebral fissure using ImageJ software (v.1.46s, National Institutes of Health, Bethesda, MD, USA); 6) corneal topography and tomography (Allegro Oculyzer®, Alcon Laboratories, Fort Worth, TX, USA) and Pentacam® optical topography assessment (Oculus Optikgerate GmbH, Wetzlar, Germany); 7) Goldmann applanation tonometry (GAT; Haag-Streit, Konig, Switzerland); and 8) ocular fundus examination. All evaluations were performed by the same examiner (ACR).
Measurement of the palpebral fissure
ImageJ is a public domain Java image processing program that can display, edit, analyze, process, save, and print images. It can also calculate the area and pixel value statistics of user-defined selections, measure distances and angles, and create density histograms and line profile plots.
An eyelid examination was performed with the eye image regis tration of patients in the primary position of gaze with a portable Sony® Cyber-shot digital camera attached to a monopod. To ensure the standardization of all measurements, photographs were taken with the participant positioned on a chin rest (for a slit-lamp) with a ruler attached at the forehead support. The same slit-lamp was used in all evaluations, thereby maintaining the same distance from the digital camera to the chin rest. The palpebral fissure height was drawn as a vertical line from the upper to the lower eyelid margin, passing through the pupil. Multiple images were captured from the study eye, and the mean measurement from the selected images was calculated.
Injection of botulinum toxin type A
Patients in the BTX-A injection group were treated with an injection of onabotulinumtoxinA (Botox®, Allergan, Irvine, CA, USA) to the palpebral portion of the orbicularis oculi muscle. Reconstitution of the drug and its application were performed by a single investi gator (MHO) according to a standardized protocol. Immediately before appli cation, each vial was reconstituted with normal saline without preservatives to a final BTX-A concentration of 50 U/mL. The participant was seated and received a total of 12.0 U of BTX-A per application, injected using an insulin syringe in equal doses into the palpebral portion of the orbicularis oculi muscle of the upper and lower eyelids. BTX-A was applied twice: after the baseline measurements, and then six months after the first injection.
Statistical analysis
The intention-to-treat principle was used in the analysis. Pre- and post-treatment analyses were conducted using the Wilcoxon signed rank test. Between-group analyses used the Mann-Whitney U-test for continuous variables and Fisher's exact test for categorical variables measured at each follow-up visit. All analyses were performed using Stata v.11 software, and p-values <0.05 were considered statistically significant. Data are presented as mean ± SD unless otherwise noted.
RESULTS
A total of 40 individuals (40 eyes) were enrolled at baseline. Three participants were lost due to voluntary non-participation in some of the follow-up visits, and so only 37 eyes of 37 participants were included for some of the follow-up analyses.
All of the eyes were graded according to the Amsler-Krumeich classification (9), based on each participant's refraction, mean central K-reading, corneal signs, and corneal thickness. Eyes were graded stage I, II, or III according to this classification.
Participant demographics
Participant demographics, study eye laterality, baseline palpebral height, corneal topography, and spherical equivalent (SE) were similar between the two study groups (Table 1).
Table 1 Comparison of baseline characteristics between the botulinum toxin-A injection and control groups
| Parametera | Control (n=21) | BTX-A (n=19) | P-value |
|---|---|---|---|
| Age, years | -25.95 (07.33) | -21.31 (07.60) | 0.052 |
| Sex, n (%) | 0.087 | ||
| Female | -11.00 (52.40) | -05.00 (26.40) | |
| Male | -10.00 (47.60) | 14.00 (73.60) | |
| Eye, n (%) | 0.357 | ||
| Right | -11.00 (52.40) | -12.00 (63.10) | |
| Left | -10.00 (47.60) | -07.00 (36.90) | |
| Palpebral fissure, mm | -09.74 (01.87) | -09.45 (01.47) | 0.560 |
| Steepest K, Pentacam, D | -53.09 (07.29) | -53.41 (05.44) | 0.714 |
| Flattest K, Pentacam, D | -49.41 (05.69) | -48.23 (05.19) | 0.645 |
| Mean K, Pentacam, D | -51.17 (06.38) | -50.67 (05.21) | 0.989 |
| Steepest K, Oculyzer, D | -53.16 (07.24) | -53.53 (05.04) | 0.694 |
| Flattest K, Oculyzer, D | -49.86 (05.88) | -48.40 (05.28) | 0.606 |
| Mean K, Oculyzer, D | -51.45 (06.45) | 50.80 (05.11) | 0.989 |
| Spherical equivalent, D | 0-9.23 (06.03) | 0-8.97 (06.12) | 0.870 |
avalues shown are mean (standard deviation), unless noted as "n (%)." BTX-A= botulinum toxin-A; K= keratometry measurements made with a pentacam® or Oculyzer® device; D= diopters.
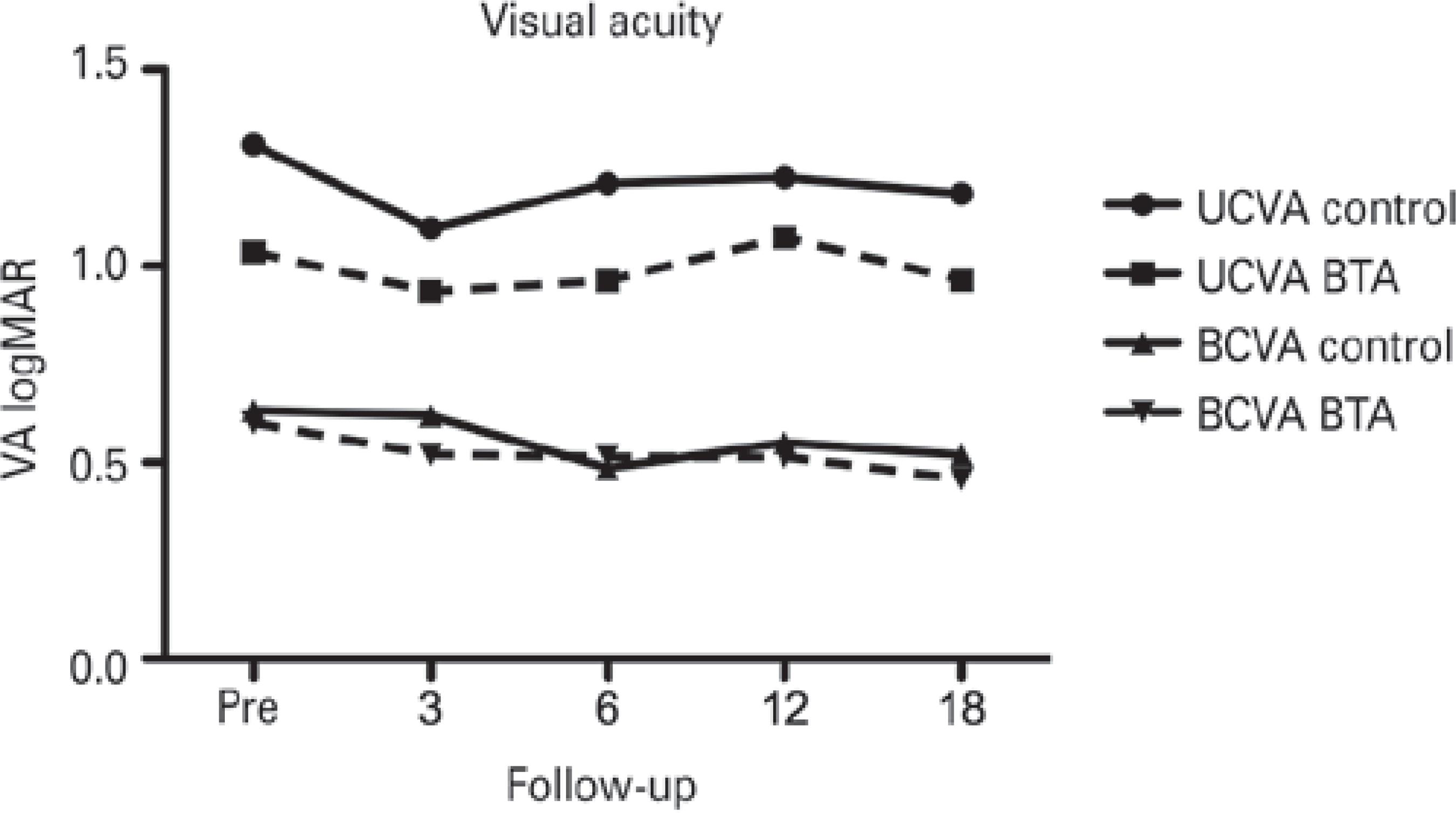
Visual acuity
Both UCVA and BSCVA, measured using a logMAR chart, were similar between the two study groups at baseline and at all follow-up time points (Figure 1.
Refractive results
The mean baseline SE was -9.23 ± 6.03 D in the control group and -8.97 ± 6.12 D in the BTX-A group (p=0.870; Tables 1 and 2). At 18 months, the mean SE was -9.70 ± 6.20 D in the control group and -8.66 ± 5.14 D in the BTX-A group, with no statistically significant difference between the groups (p=0.583; Table 2).
Table 2 Comparison of the spherical equivalent measurements between the botulinum toxin-A injection and control groups
| Parameter, mean (SD) | Baseline | 3 months | 6 months | 12 months | 18 months |
|---|---|---|---|---|---|
| Spherical equivalent, D | |||||
| Control | -9.23 (6.03) | -9.51 (6.03) | -9.60 (6.13) | -9.80 (6.17) | -9.70 (6.20) |
| BTX-A | -8.97 (6.12) | -8.94 (5.90) | -8.85 (5.89) | -8.71 (5.70) | -8.66 (5.14) |
| P-value | 0.870 | 0.796 | 0.663 | 0.564 | 0.583 |
SD= standard deviation; D= diopters; BTX-A= botulinum toxin-A.
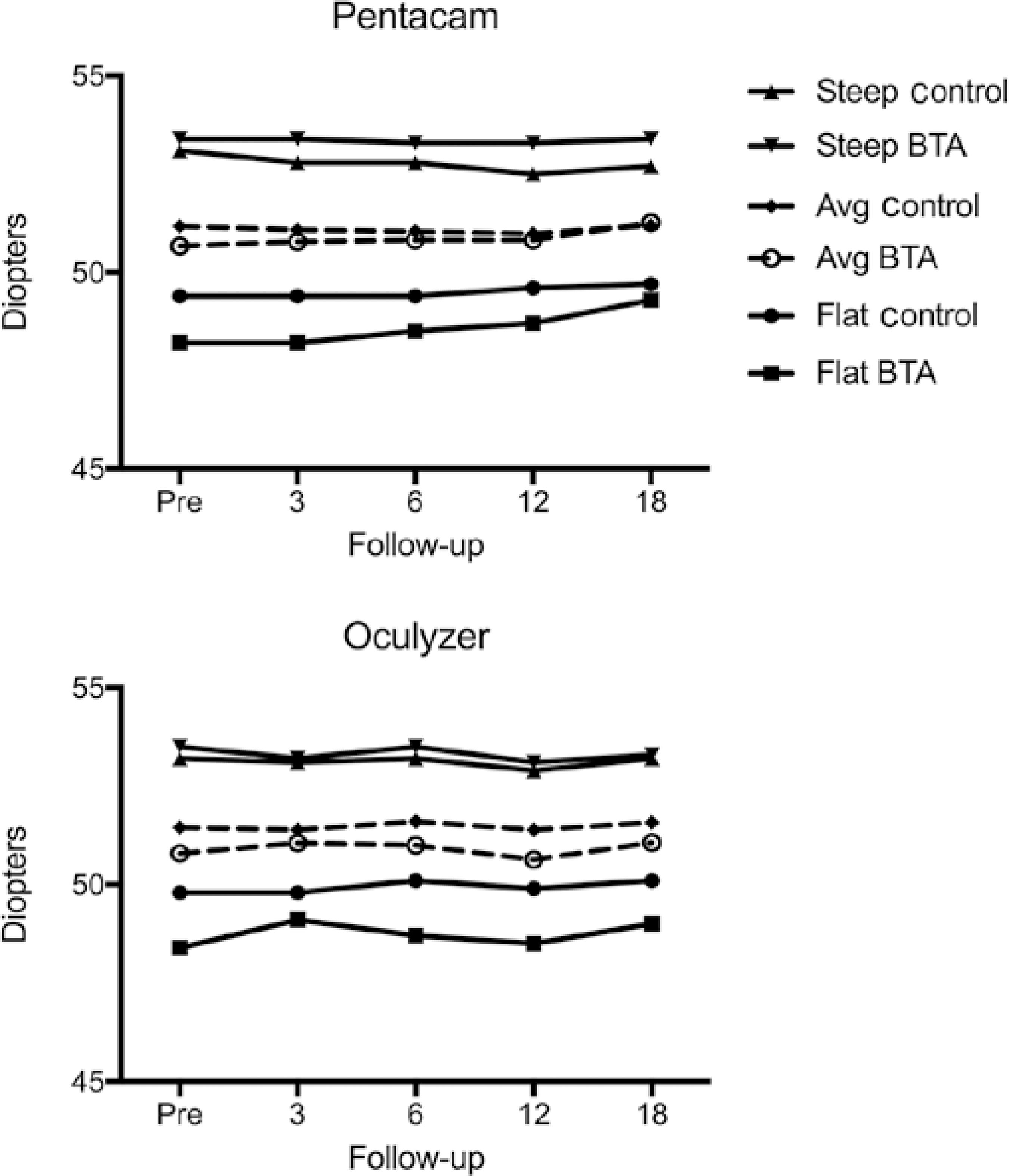
Topographic results
After adjusting for baseline levels, there was no statistically significant difference between the groups at the 18-month follow-up for all three topography parameters assessed with the Pentacam® (flattest keratometry (K1), p=0.795; steepest keratometry (K2), p=0.562; and mean keratometry (Km), p=0.903), and with the Oculyzer® (K1, p=0.783; K2, p=0.783; and Km, p=0.842) (Figure 2).
Palpebral fissure
The mean height of the palpebral fissure at baseline was 9.74 ± 1.87 mm in the control group and 9.45 ± 1.47 mm in the BTX-A group (p=0.560; Tables 1 and 3). At 18 months, the respective palpebral fissure measurements were similar at 10.0 ± 1.49 mm and 9.62 ± 1.73 mm in the control and BTX-A groups, respectively (p=0.337; Table 3).
Table 3 Comparison of the heights of the palpebral fissure between the botulinum toxin-A injection and control groups
| Parameter, mean (SD) | Baseline | 3 months | 6 months | 12 months | 18 months |
|---|---|---|---|---|---|
| Palpebral fissure, mm | |||||
| Control | 9.74 (1.87) | 9.14 (1.26) | 9.84 (1.19) | 9.66 (1.75) | 10.06 (1.49) |
| BTX-A | 9.45 (1.47) | 9.79 (1.28) | 9.82 (1.51) | 9.84 (1.59) | 0 9.62 (1.73) |
SD= standard deviation; BTX-A= botulinum toxin A.
Pachymetry results
Table 4 shows the pachymetry results at the central and thinnest parts of the corneas. There was no statistically significant difference between the two study groups at 18 months of follow-up in any parameter (p=0.669 and p=0.464 for the central and thinnest corneal regions measured by Pentacam® pachymetry, and p=0.748 and p=0.502 for the central and thinnest areas measured by Oculyzer® pachymetry).
Table 4 Comparison between the botulinum toxin-A injection and control groups of corneal pachymetric measurements made using the Pentacam® and Oculyzer® devices
| Parameter, mean (SD) | Baseline | 3 months | 6 months | 12 months | 18 months |
|---|---|---|---|---|---|
| Pentacam® | |||||
| Central position, µm | |||||
| Control | 447.7 (47.6) | 451.4 (42.7) | 451.0 (50.3) | 445.1 (46.4) | 447.0 (47.9) |
| BTX-A | 460.4 (45.7) | 447.1 (48.7) | 464.1(45.0) | 453.4 (60.0) | 454.7 (53.9) |
| Thinnest position, µm | |||||
| Control | 419.6 (53.0) | 421.8 (44.2) | 423.5 (55.9) | 416.0 (51.1) | 418.5 (52.2) |
| BTX-A | 433.1 (46.9) | 430.0 (48.9) | 437.8 (45.6) | 431.1 (57.3) | 425.6 (52.4) |
| Oculyzer® | |||||
| Central position, µm | |||||
| Control | 461.9 (46.4) | 464.4 (46.8) | 464.0 (50.0) | 459.7 (52.5) | 463.4 (47.8) |
| BTX-A | 469.6 (49.1) | 473.9 (53.8) | 471.4 (59.3) | 466.3 (66.1) | 459.5 (63.4) |
| Thinnest position, µm | |||||
| Control | 430.7 (55.1) | 430.7 (54.4) | 432.4 (52.6) | 430.7 (54.7) | 433.6 (49.8) |
| BTX-A | 439.8 (51.9) | 443.6 (52.4) | 442.4 (53.7) | 432.6 (67.6) | 432.5 (63.1) |
SD= standard deviation; BTX-A= botulinum toxin-A.
Tonometry results
The tonometry data were evaluated by GAT. Intraocular pressure (IOP) did not differ significantly between the groups at 18 months (p=0.322; Table 5).
Table 5 Comparison of Goldmann applanation tonometry measurements between the botulinum toxin-A injection and control groups
| Parameter, mean (SD) | Baseline | 3 months | 6 months | 12 months | 18 months |
|---|---|---|---|---|---|
| GAT, mmHg | |||||
| Control | 9.1 (2.3) | 8.8 (2.5) | 8.9 (2.7) | 9.3 (3.0) | 9.1 (1.8) |
| BTX-A | 9.3 (2.2) | 8.4 (2.5) | 8.6 (2.5) | 7.6 (2.4) | 8.3 (3.1) |
SD= standard deviation; GAT= Goldmann applanation tonometry; BTX-A= botulinum toxin A.
DISCUSSION
Various factors can result in the deterioration of keratoconus, including ultraviolet-B light, atopy, mechanical eye rubbing, and improperly fitting contact lenses (2). Researchers have studied genetic, histopathologic, biomechanical, and inflammatory aspects of the disease to elucidate keratoconus etiology and improve the currently available treatments (10,11). Elevated tension within the eyelid can be transmitted to the juxtaposed corneal surface, thereby altering the corneal topography (5,12); thus, reducing the muscular force of the eyelid may result in changes to corneal measurements in keratoconus patients. With the goal of developing new treatment options for keratoconus, the present study evaluated whether BTX-A injection into the orbicularis oculi muscles of keratoconus patients improved corneal topographic parameters by reducing the eyelid muscular forces that are translated to the ocular surface and exacerbate symptoms.
However, the administration of two BTX-A doses over 6 months resulted in no changes to visual acuity and refraction in our keratoconus cohort. Previous studies have reported no success in treating keratoconus by cross-linking corneal collagen matrices to stabilize the ocular tissue (13,14), an approach that also brought no changes in UCVA, BSCVA, SE, or keratometry. Another keratoconus intervention involves implanting plastic intracorneal ring segments to stabilize the cornea, but this brings the risk of ocular complication, requiring ca reful selection of patients appropriate for the procedure (2,15,16). Several factors contribute to the development of corneal astigmatism, including age, sex, tear film insufficiency, IOP, and physical pressure exerted by the eyelids (17). In a 2015 report by members of our research team, the magnitude of astigmatism was significantly reduced and palpebral fissure height significantly increased in the affected eyes of hemifacial spasm patients treated with BTX-A (18). In that study, BTX-A treatment temporally alleviated eyelid tension, thereby potentially reducing the transmission of physical forces from the eyelids to the cornea. In the present study, no statistically significant differences were observed in the corneal topographic parameters and palpebral fissure dimensions of keratoconus eyes during the 18 months following BTX-A administration. Usually, BTX-A effects appear within 1-2 days, peak at 1-2 weeks, and gradually decline after 3-6 months, depending on the patient's clinical condition (8). We observed no differences in palpebral fissure measurements 3 months after the first and second BTX-A applications, probably because the tension exerted by the eyelid on the cornea in keratoconus patients is not as high as it is in hemifacial spasm patients. Thus, BTX-A application to the orbicularis oculi muscles of keratoconus patients was not associated with significant changes in corneal astigmatism or palpebral fissure dimensions.
Methodologies for evaluating eyelid tension are not standardized and vary between studies, complicating the comparison of results from different groups. The present study indirectly evaluated the reduction in eyelid muscular force due to BTX-A application to the orbicularis oculi muscles by studying the subsequent effects on the corneal topographic parameters. Other research groups have described using a lid tensiometer instrument to assess the generation of eyelid muscular force (19,20). One report indicated that, under normal circumstances, eyelid tension was unlikely to adversely affect the shape and power of the cornea (19). In addition, it has been noted that no difference in lid tension exists between Asians and Caucasians (20) even though Asians are at increased predisposition to developing keratoconus. In contrast, studies that evaluated corneal astigmatism changes after BTX-A injection in patients with blepharospasm concluded that eyelid-derived forces may play an important role in modifying corneal curvature (17,21).
The pachymetry results for the central and thinnest regions of the cornea remained similar between the groups throughout the 18 months of follow-up. This is not surprising, because we did not administer BTX-A directly into the corneal tissue. Pachymetry was assessed using two different devices, but both were based on the same principle (Scheimpflug tomography). This approach has good reproducibility in differentiating normal corneas from subclinical and symptomatic keratoconus cases (22).
GAT is the gold standard for tonometry and is the most frequently used technique for IOP measurement. Eyes with corneal pathologies can present challenges for measuring IOP because of changes to the corneal thickness, rigidity, and curvature (23). However, newer tonometers such as the Pascal dynamic contour tonometer (Ziemer Ophthalmic Systems AG, Bern, Switzerland) and the Ocular Response Analyzer and TonoPen XL (Reichert Ophthalmic Instruments, Depew, NY, USA) have better diagnostic accuracy than GAT for keratoconic corneas (23).
We are not aware of any previous reports that have used BTX-A to reduce eyelid muscular force in patients with keratoconus. Although several studies (17-20) have confirmed the influence of eyelid tension on corneal shape, it appears that eyelid tension in keratoconus patients is no higher than for other conditions such as hemifacial spasm and blepharoptosis (18,24). In the present study, BTX-A application did not result in a significant change to corneal parameters. It remains unclear whether BTX-A application to the orbicularis oculi muscles could be a useful adjuvant approach for controlling the progression of keratoconus. Corneal changes after the application of BTX-A in orbicularis oculi muscles, if there are any, take longer to be detected than earlier eyelid morphometric changes (18); our follow-up period may therefore not have been long enough to detect associated corneal changes in patients with keratoconus. Furthermore, keratoconic corneas may present structural alterations that respond differently from the corneas of patients with hemifacial spasm.
We recognize potential limitations in this study. The number of BTX-A applications was limited, and the follow-up period was rela tively short. Future studies of keratoconus patients with a longer follow-up period and an increased number of BTX-A applications may help to elucidate whether BTX-A application in the orbicularis oculi muscle could be a valuable adjuvant treatment to help control the progression of keratoconus to some degree.
Keratoconus etiology and pathogenesis mechanisms remain unclear. The recent Global Consensus on Keratoconus and Ectatic Di seases (25) concluded that keratoconus was a multifactorial disease with genetic, biochemical, biomechanical, and environmental components. The panelists found that the most important objectives of nonsurgical treatment were to halt progression and bring about visual rehabilitation.
The past two decades, in particular, have seen exciting new de velopments that, for the first time, promise to alter the natural history of keratoconus favorably to benefit patients. Scientific interest in keratoconus is likely to remain high in the foreseeable future, to avoid iatrogenic ectasia, unmask subclinical disease, and expand the rapeutic options (26).
In conclusion, BTX-A administration to the orbicularis oculi muscles of keratoconus patients did not cause detectable changes in corneal parameters secondary to reduced eyelid muscular force during 18 months of follow-up.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

