INTRODUCTION
The intravitreal administration of vascular endothelial growth factor inhibitors (anti-VEGF) has had positively effects on the visual prognosis of patients with neovascular age-related macular degeneration (AMD). The effectiveness of monthly intravitreal ranibizumab injections was demonstrated previously in the multi-center Marina(1) and ANCHOR(2) studies. Stable visual acuity has been provided to many patients through monthly or pro-re-nata treatment protocols that were designed around the patient and disease severity(3). However, although many patients achieved positive results at treatment onset, some patients continue to experience macular exudation and progressive vision loss despite receiving repeated injections in subsequent months. The 1- and 2-year results of the CATT study showed that in more than 50% of patients who received ranibizumab treatment, persistent fluid development occurred at the end of month 12(4,5). Patients in this group, in whom dramatic disease progression was observed, were defined as the treatment-resistant patient group. Although the mechanism of resistance could not be entirely explained, tolerance to ranibizumab and tachyphylaxis are thought to contribute to this situation(6). Although no dosage-related differences in the efficiency of ranibizumab were observed in the Harbor study(7), the SAVE studies(8,9) showed that a loss of efficacy could be overcome in some patients by increasing the dosage and administration frequency. However, the remaining patients did not achieve positive results, leading to the clinical outcome of permanent vision loss.
Recently published studies have discussed the effectiveness of intravitreal aflibercept in cases involving insufficient responses to ranibizumab treatment and the development of recurrence and tolerance in subsequent months(10-20). Additional studies have shown that a change of intravitreal agents might be effective in patients with neovascular AMD cases who develop tachyphylaxis to anti-VEGFs(21,22). In contrast to previous studies, we aimed to demonstrate the short-term effectiveness of intravitreal aflibercept therapy in patients who had exhibited complete resistance to ranibizumab treatment; in other words, we included patients who were completely non-responsive from the first dose, as well as those who developed tachyphylaxis to ranibizumab. The present study compared these two groups.
METHODS
This interventional clinical study included 44 eyes of 38 patients with exudative AMD and complete ranibizumab (Lucentis®, Genentech, Inc., South San Francisco, CA, USA) resistance or ranibizumab tachyphylaxis and subsequently received three aflibercept (Eylea®, Regeneron, Tarrytown, NY, USA) injections and were followed up for 3 months. This study was conducted in accordance with the tenets of the Declaration of Helsinki, after receiving approval from the institutional ethics review board.
Patient selection
The charts of all patients with exudative AMD who were treated with ranibizumab at the Gulhane Military Medical Academy, Department of Ophthalmology were reviewed, and the above-mentioned patients and eyes were subsequently selected. A diagnosis of exudative AMD was confirmed by fluorescein angiography and optical coherence tomography (OCT). Patients were divided into "complete resistance" (23 eyes) and "tachyphylaxis" groups (21 eyes). Complete ranibizumab resistance was defined as a decrease in the central subfield thickness (CST) of <30 μm, followed by persistent intraretinal and/or subretinal fluid despite monthly injections. Recurrences that responded well to repeated ranibizumab injections were not considered resistant. Ranibizumab tachyphylaxis was defined as an initial decrease of >30 μm in the CST and subretinal fluid after the first injection, followed by a persistent increase in the CST or persistent subretinal fluid on OCT despite repeated injections. Eyes were considered to be completely resistant or tachyphylactic after six monthly ranibizumab injections. The OCT criteria for aflibercept treatment were identical to the ranibizumab re-treatment criteria. Indications for aflibercept treatment included persistent subretinal/intraretinal fluid with or without pigment epithelial detachment (PED).
Inclusion criteria
Prior to the initiation of 2 mg/0.05 mL aflibercept therapy, eyes with persistent subfoveal fluid must have received six monthly injections of 0.5-mg ranibizumab. The required interval between the last ranibizumab treatment and first aflibercept treatment was >4 weeks and <6 weeks(17).
Exclusion criteria
Eyes previously treated with photodynamic therapy or intravitreal bevacizumab, eyes with insufficient clinical records or <6 previous ranibizumab injections, eyes with a lack of follow-up after conversion to aflibercept, eyes with macular dryness, eyes that responded well to repeated ranibizumab injections, and eyes with a diagnosis of polypoidal choroidal vasculopathy, retinal angiomatous proliferation, retinal pigment epithelium tear, fibrovascular scarring, or retinal conditions other than exudative AMD were not included in the study.
Main outcome measures
The main outcome measures were the visual and anatomical results at 1 month after the third injection of aflibercept. Best corrected visual acuity (BCVA) was measured using the Snellen visual acuity chart. BCVA values were converted to the logarithm of minimum angle of resolution (logMAR) format for statistical analysis(23). With aflibercept treatment, improvement and worsening were defined as a gain or loss of ≥2 lines on the Snellen chart. Stabilization was defined as remaining within ±1 line of the baseline Snellen result after the last dose of ranibizumab(18). Anatomical changes in CST were classified as a complete resolution or macular dryness (absence of both subretinal and intraretinal fluid on all radial OCT scans), partial resolution, unchanged, or worsening(18,24). Spectral domain OCT images were obtained using a Heidelberg Spectralis OCT (Heidelberg Engineering, Inc., Vista, CA, USA). All measurements were recorded at the baseline and after each intravitreal ranibizumab and aflibercept injection. Calipers were used in instances involving artifacts in the automated measurements. Pigment epithelium detachment (PED) was categorized as present or absent based on a review of subretinal OCT scans.
Statistical analysis
Data are presented as means ± standard deviations or frequencies. The Shapiro-Wilk test was used to evaluate the normality of measurements. Paired variables before and after aflibercept switching were analyzed using the Wilcoxon signed rank test. Fisher's exact test, the χ2 test, and McNemar's test were used to assess categorical variables where appropriate. The Mann-Whitney U test was used to compare improvements between the ranibizumab resistance and tachyphylaxis groups. Improvements were calculated by subtracting pre-treatment data from post-treatment data. All statistical tests were two-tailed, and significance was defined as a p value <0.05. Statistical analyses and graphic production were performed using MedCalc (Version 15.4; MedCalc Software, Ostend, Belgium).
RESULTS
Among the 23 eyes with complete ranibizumab resistance and 21 eyes with ranibizumab tachyphylaxis, the mean ages at baseline were 73.4 ± 10.5 and 72.1 ± 8.8 years, respectively (p=0.54). All eyes received three aflibercept treatments after a series of six ranibizumab injections. None of the studied eyes developed endophthalmitis, sterile inflammation, vitreous hemorrhage, or retinal tears.
Anatomic outcomes with aflibercept
Figure 1 A shows the changes in mean CST over the study period relative to the baseline values. In both groups, the CST decreased significantly after each injection of aflibercept as compared to the values measured after the last ranibizumab treatment (p≤0.002 for all; Figure 2 A, B, C). There was no significant difference between the groups regarding the decrease in CST after each of the three aflibercept injections when compared with the values measured after the last ranibizumab treatment (p≥0.13 for all; Figure 3 A).
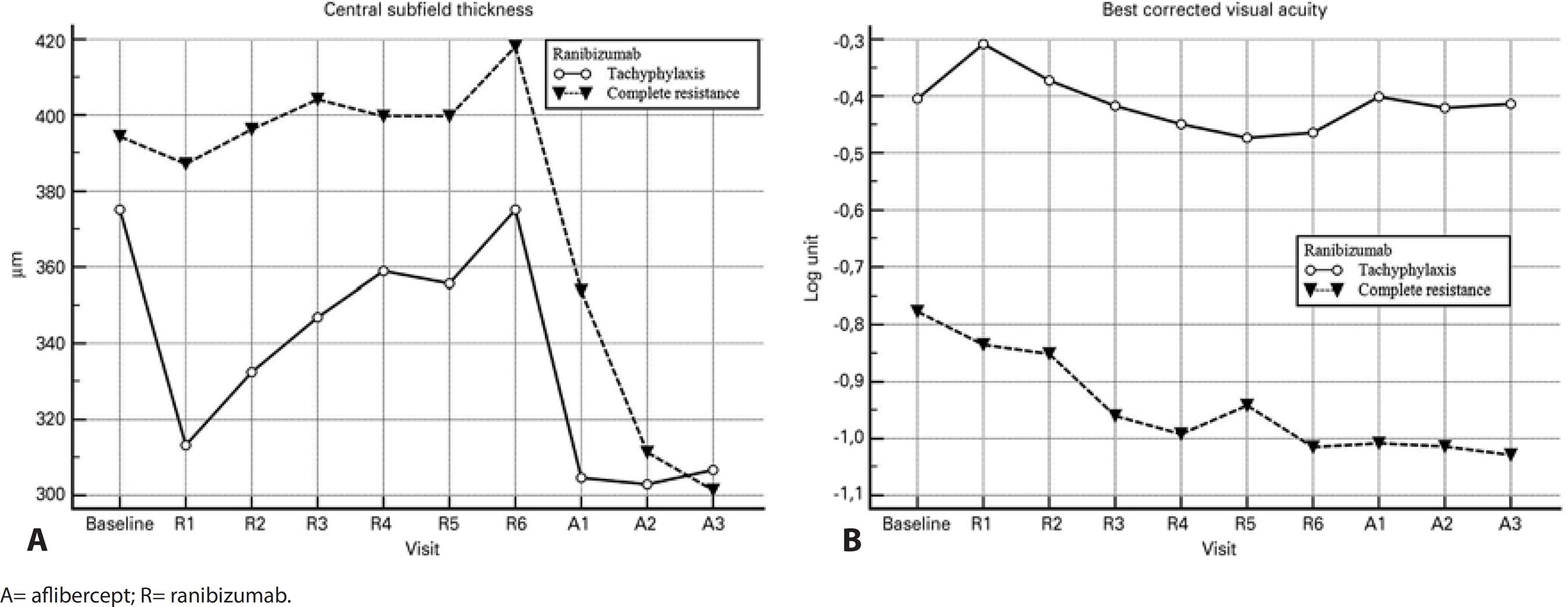
Figure 1 Graphs of the changes in visual and anatomical results over time. A) Changes in the central subfield thickness. B) Changes in the best corrected visual acuity.
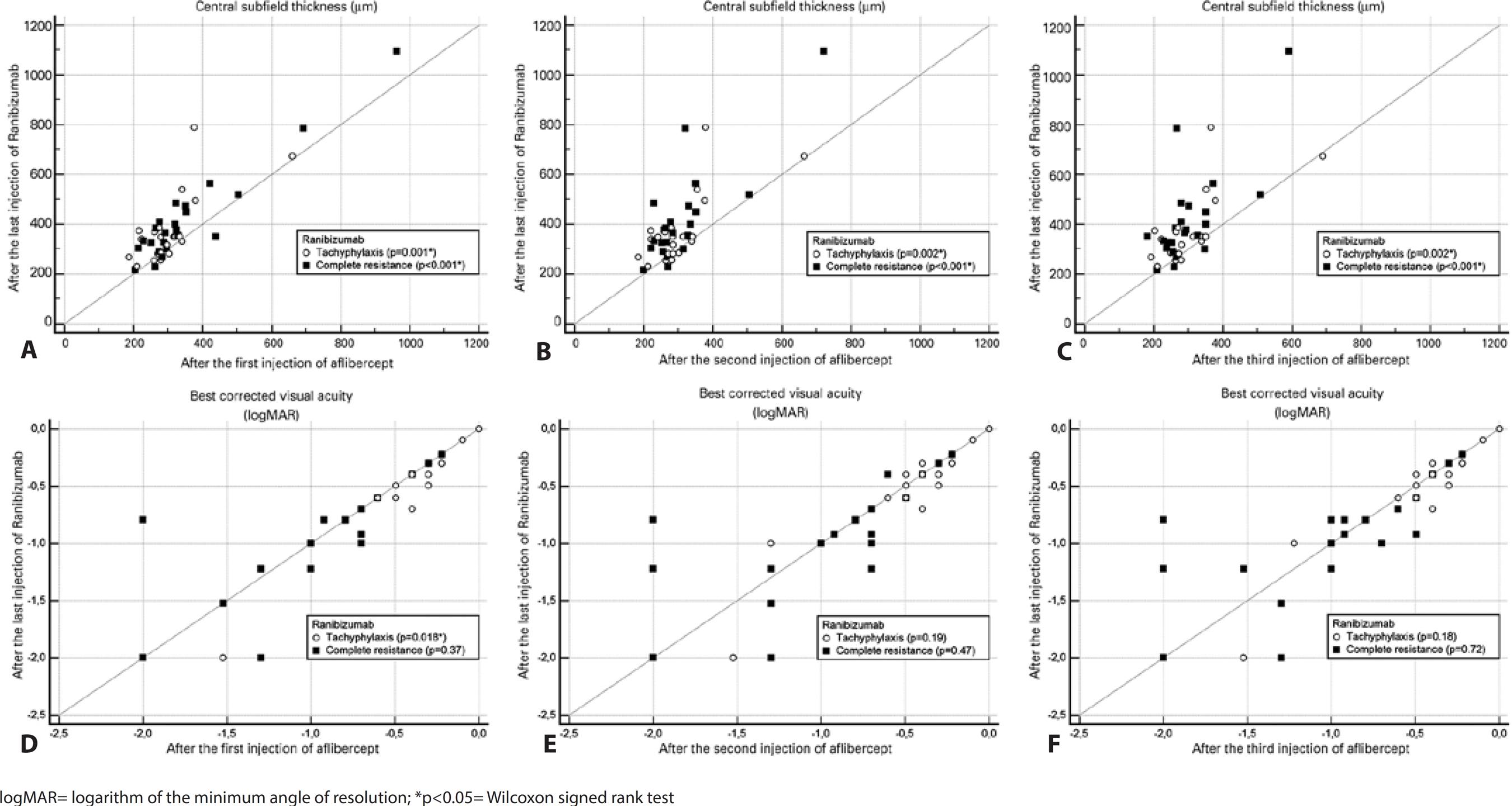
Figure 2 Scatterplots showing the results after each aflibercept injection relative to those at the last visit after ranibizumab treatment. A) Central subfield thickness (CST) after the first injection. B) CST after the second injection. C) CST after the third injection. D) logMAR visual acuity after the first injection. E) logMAR visual acuity after the second injection. F) logMAR visual acuity after the third injection.
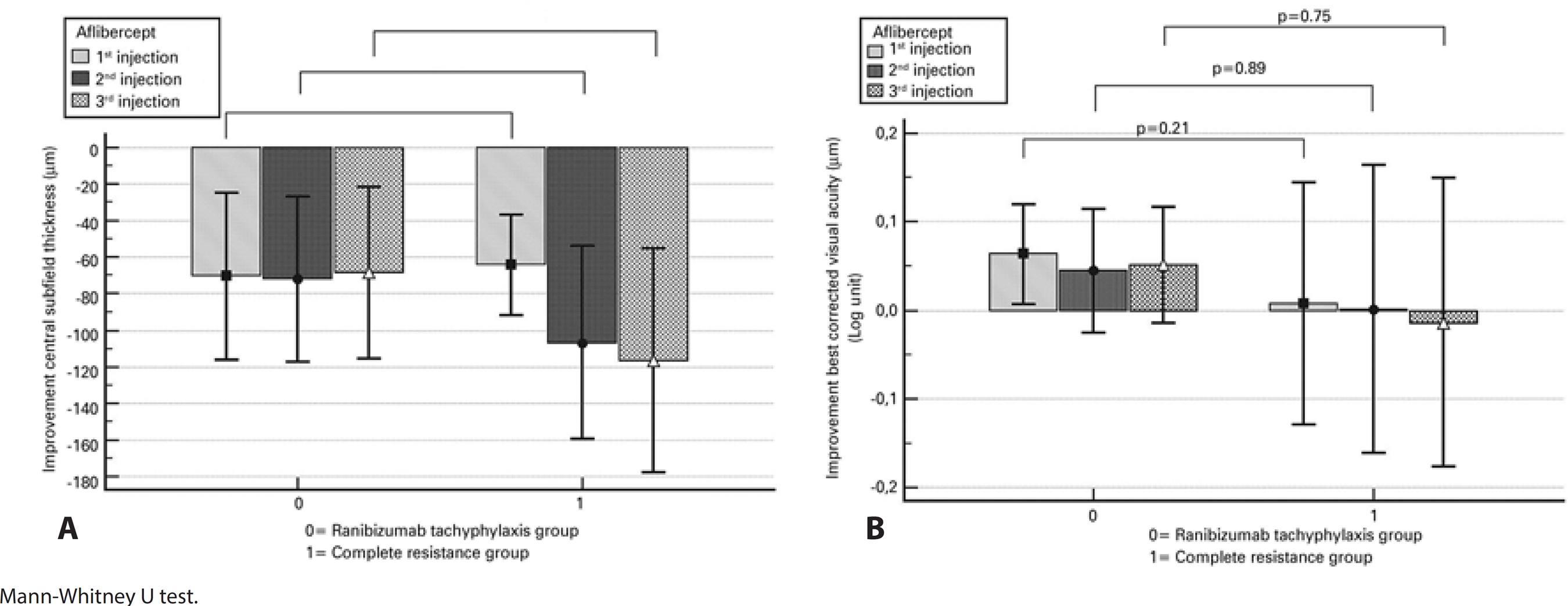
Figure 3 Graphs showing the visual and anatomical improvements following each aflibercept injection relative to the values at the last visit after ranibizumab treatment. A) Improvements in the central subfield thickness. B) Improvements in the best corrected visual acuity.
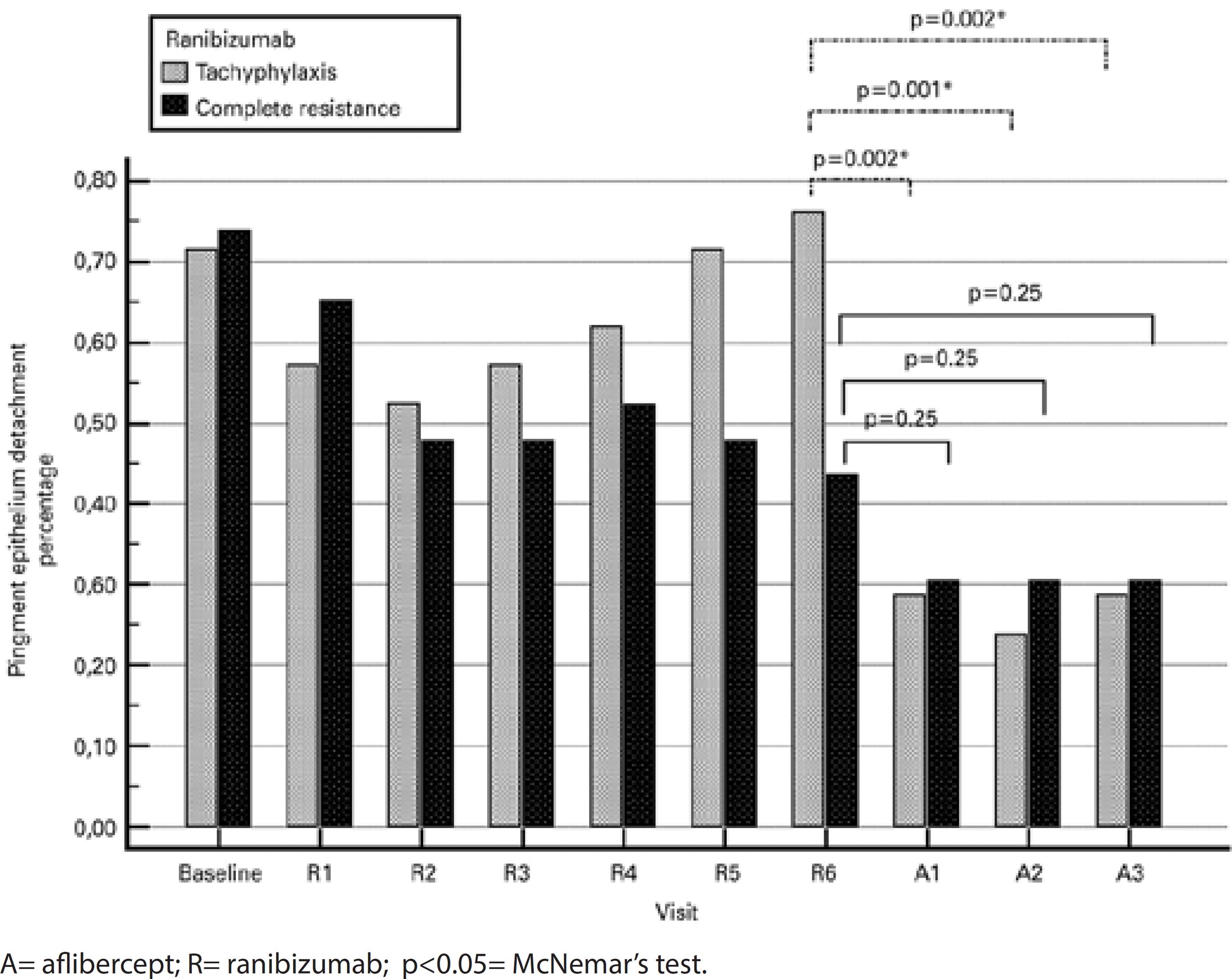
After three injections, eight (38.1%) of the 21 eyes in the tachyphylaxis group had a dry macula, six (28.6%) had partial resolution of subretinal fluid, three (14.3%) remained unchanged, and four (19%) worsened (Table 1). The mean improvement in CST was 68.4 μm (range: 22-425 μm, p=0.002; Figure 3 A). In 16 of 21 eyes (76.2%), a subfoveal PED was observed at the visit after the last ranibizumab injection. In contrast, a subfoveal PED was observed in six eyes during the visit after the third aflibercept injection (28.6%; p=0.002; Figure 4).
Table 1 Distributions of visual and anatomical improvements following each aflibercept injection relative to the values after the last ranibizumab treatment
| Final outcome measures | Ranibizumab tachyphylaxis (n=21) |
Complete resistance (n=23) |
P value |
|---|---|---|---|
| Best corrected visual acuity | |||
| After 1st aflibercept injection n (%) | |||
| Gaining two snellen lines | 2 ( 9.5) | 0 (0) | 0.22a |
| Gaining one snellen line | 5 (23.8) | 5 (21.7) | 1.00a |
| Unchanged | 14 (66.7) | 14 (60.9) | 0.69b |
| Losing one snellen line | 0 (0) | 3 (13.0) | 0.23a |
| Losing two snellen lines | 0 (0) | 1 ( 4.3) | 1.00a |
| After 2nd aflibercept injection | |||
| Gaining two snellen lines | 2 ( 9.5) | 1 ( 4.3) | 0.59a |
| Gaining one snellen line | 6 (28.6) | 6 (26.1) | 0.85b |
| Unchanged | 10 (47.6) | 12 (52.2) | 0.76b |
| Losing one snellen line | 3 (14.3) | 2 ( 8.7) | 0.65a |
| Losing two snellen lines | 0 (0) | 2 ( 8.7) | 0.48a |
| After 3rd aflibercept injection | |||
| Gaining two snellen lines | 2 ( 9.5) | 1 ( 4.3) | 0.59a |
| Gaining one snellen line | 7 (33.3) | 7 (30.4) | 0.83b |
| Unchanged | 9 (42.9) | 10 (43.5) | 0.96b |
| Losing one snellen line | 3 (14.3) | 4 (17.4) | 1.00a |
| Losing two snellen lines | 0 (0) | 1 ( 4.3) | 1.00a |
| Central subfield thickness | |||
| After 1st aflibercept injection n (%) | |||
| Dry macula | 6 (28.6) | 5 (21.7) | 0.60b |
| Partial resolution | 7 (33.3) | 10 (43.5) | 0.49b |
| Unchanged | 6 (28.6) | 5 (21.7) | 0.60b |
| Worse | 2 ( 9.5) | 3 (13.0) | 1.00a |
| After 2nd aflibercept injection n (%) | |||
| Dry macula | 8 (38.1) | 8 (34.8) | 0.82b |
| Partial resolution | 5 (23.8) | 9 (39.1) | 0.27b |
| Unchanged | 4 (19.0) | 4 (17.4) | 1.00a |
| Worse | 4 (19.0) | 2 (08.7) | 0.40a |
| After 3rd aflibercept injection n (%) | |||
| Dry macula | 8 (38.1) | 9 (39.1) | 0.94b |
| Partial resolution | 6 (28.6) | 9 (39.1) | 0.46b |
| Unchanged | 3 (14.3) | 3 (13.0) | 1.00a |
| Worse | 4 (19.0) | 2 ( 8.7) | 0.40a |
aFisher’s exact test (two-sided);
bPearson chi-square test.
After three injections, nine (39.1%) of the 23 eyes in the complete resistance group had a dry macula, nine (39.1%) had partial resolution of subretinal fluid, three (13.0%) remained unchanged, and two (8.7%) worsened (Table 1). The mean improvement in CST was 116.6 μm (range: 45-520 μm, p<0.001; Figure 3 A). In 10 of the 23 eyes (43.5%), a subfoveal PED was observed at the visit after the last ranibizumab injection. In contrast, a subfoveal PED was observed in seven eyes at the visit after the third aflibercept injection (30.4%; p=0.25; Figure 4).
Visual outcomes with aflibercept
The change in mean BCVA during the study period was compared with the baseline values and is shown in figure 1 B. After the first injection of aflibercept, the mean visual acuity improved significantly in the tachyphylaxis group (p=0.018) but remained unchanged in the complete resistance group (p=0.37; Figure 2 D). There was a trend towards improved mean visual acuity in both groups after the second and third injections when compared to the last visit after ranibizumab treatment, although these improvements were not statistically significant (Figure 2 E, F). There was no significant difference between the groups regarding BCVA improvements after each of the three injections of aflibercept when compared to those at the last visit after ranibizumab treatment (p≥0.21 for all; Figure 3B).
After three injections, two (9.5%) of the 21 eyes in the tachyphylaxis group gained ≥2 Snellen lines, 19 (90.5%) remained stable, and none (0%) lost ≥2 Snellen lines (Table 1). The mean increase in the logMAR VA was 0.05 (range: -0.22-0.47, p=0.18; Figure 3 B).
After three injections, one (4.3%) of 23 eyes in the complete resistance group gained ≥2 Snellen lines, 21 (91.4%) remained stable, and one (4.3%) lost ≥2 Snellen lines (Table 1). The mean gain in the logMAR VA was -0.013 (range: -1.20-0.69, p=0.72; Figure 3 B).
DISCUSSION
For patients with neovascular AMD who achieved an insufficient clinical response despite receiving a suitable treatment dosage, the response to a second anti-VEGF agent differs from patient to patient. Variable resistance mechanisms among patients comprise an important cause of these differences(11). According to a previous report, 2% of patients receiving treatment for neovascular AMD will develop ranibizumab tachyphylaxis(22). In published studies, tachyphylactic, tolerant, and recurrent cases are generally considered resistant. In tachyphylaxis, which differs from tolerance and recurrence, subretinal fluid is maintained or increases despite recurring intravitreal injections(22). Therefore, cases wherein visual stabilization is achieved through intravitreal treatment and wherein the CST has not changed are not considered to be solely tachyphylactic(21,22). Tachyphylaxis is believed to be caused by the acute development of antibodies against anti-VEGF agents, and usually occurs after the first dose. It is also believed that while receiving ranibizumab treatment, which has a specific affinity for VEGF-A, an alternative, VEGF-B-dependent angiogenic process affects the development of tachyphylaxis(25). The high affinity of aflibercept for both VEGF-A and VEGF-B could therefore explain the effectiveness of this agent in tachyphylaxic cases. Although no studies have selectively investigated such cases, Hariri et al. very recently published the results of a short-term investigation of intravitreal aflibercept in cases exhibiting resistance to intravitreal ranibizumab and bevacizumab. These authors observed stabilization or recovery of the subretinal fluid in 87% of the cases(26). Similarly, our study observed corresponding rates of 81% in the tachyphylaxis group and 91.3% in the complete resistance group.
In our study, visual acuity stabilization was achieved in 90.5% and visual acuity increased in 9.5% of eyes with ranibizumab tachyphylaxis after three doses of aflibercept injections; among eyes with complete resistance to ranibizumab, visual acuity stabilization was observed in 91.4% and visual acuity was increased in 4.3%. These functional results were similar to those of a retrospective study by Ho et al. in which the importance of tachyphylaxis in resistance was emphasized using an average of 2.6 aflibercept injections that provided visual stabilization in 85% and increased visual acuity in 7% of eyes(18). That study also observed full resolution of subretinal/intraretinal fluid in 35-42% of the eyes and a partial response in 18-25% of the eyes; the corresponding results in our study were found to be 38.1% and 23.8% in the tachyphylaxis group, respectively, and 39.1% and 39.1% in the complete resistance group, respectively. Although the anatomical and functional results of our study were similar to those reported by Ho et al., the latter study involved cases resistant to bevacizumab as well as those resistant to ranibizumab, and the previous average injection amounts varied rather widely; in other words, our study was more homogeneous.
In our study, CST decreased in 66.7% of the tachyphylaxis group and 78.2% of the complete resistance group after three aflibercept injections. These results were lower than the 91% decrease observed in a 4-month retrospective study of 68 ranibizumab-resistant eyes conducted by Yonekawa et al(20). However, the increase in visual acuity at the end follow-up in that study was not statistically significant, similar to the results of our study.
In our study, the decreases in CST at the end of 3 months were 68.4 μm in the tachyphylaxis group and 116.6 μm in the complete resistance group. These amounts were similar to the 89.4-μm decrease observed in a prospective 6-month study of a similar patient cohort by Chang et al., and the 87.2-μm decrease observed in a retrospective 6-month study by Kumar et al.(3,16). In the study by Chang et al., an increase in visual acuity of ≥1 Snellen line was detected in 55% of the eyes at 6 months; in our study, the corresponding rates at the end of 3 months were 42.8% in the tachyphylaxis group and 34.7% in the complete resistance group. Notably, the increase in visual acuity observed by Chang et al. at the end of a 6-month follow-up was statistically significant, whereas in our study, the change at the end of 3 months was not significant. Kumar et al. observed a statistically significant average change of 0.02 logMAR at the end of the follow-up period, whereas in our study the corresponding rates were 0.05 logMAR in the tachyphylaxis group and -0.01 logMAR in the complete resistance group. This difference was not statistically significant. However, the different natures, follow-up periods, numbers of injections, and patient groups of these studies might have led to similar rates with statistical variation.
A retrospective study by Arcinue et al., one of the most recent studies on this subject, observed an average visual acuity increase of 0.05 logMAR together with a dry macula in 60.3% of the eyes at the end of 6 months(15). Additionally, visual acuity remained unchanged in 38.1% of the eyes, an increase of ≥1 line was observed in 36.5% of the eyes, and a decrease of ≥1 line was observed in 23.8% of the eyes. In contrast, in our study, the dry macula and unchanged, increased, and decreased visual acuity rates at 3 months after the aflibercept injections were 38.1%, 42.9%, 42.8%, and 14.3%, respectively, in the tachyphylaxis group, and 39.1%, 43.5%, 34.7%, and 21.7%, respectively, in the complete resistance group. The high rate of dry macula in the study by Arcinue et al. can be largely attributed to the inclusion of both recurrent and persistent cases in the working group(15).
Studies have shown that intravitreal aflibercept injections are also effective against other anti-VEGF resistant PEDs(3,27). In this regard, Singh et al. conducted the prospective ASSESS study, which included a follow-up period of 12 months; at the end of 6 months, PED regression was observed in 19.2% of the studied eyes(19). In our study, at the end of 3 months, PED had completely regressed in 13.1% of the eyes that were completely resistant to ranibizumab; in addition, the very high PED regression rate of 47.6% in the tachyphylaxis group was one of the most significant findings of our study. As specifically noted in the ASSESS study, a change in the intravitreal agent yields a much higher anatomic response among patients who have developed anti-VEGF resistance than in other patient groups because of tachyphylaxis(19). However, given the various issues with the identification of patients who develop tachyphylaxis in isolation, such differences in response are difficult to determine selectively(19). Consequently, our results are very important because this is the first study to selectively evaluate eyes with ranibizumab tachyphylaxis and eyes with complete resistance to ranibizumab, which were not grouped in previous studies. In addition, similar to the previous studies in the literature, we observed a disagreement between anatomic and functional improvements. This discrepancy can be attributed to the loss of photoreceptors and functional losses in the subretinal area during the early period of the disease(11). Studies with much longer terms and those that include only tachyphylactic patients are needed to determine whether this positive anatomic effect of intravitreal aflibercept injection would be maintained during a longer follow-up, particularly in the ranibizumab tachyphylaxis group.
This study had some limitations. We note that the study design, short follow-up term, and relatively small sample might have influenced our results. In addition, Snellen and logMAR are not the best options for BCVA measurement in retinal studies. Rather, ETDRS letters should be used. Although a diagnosis of tachyphylaxis is mainly based on clinical observations, some patients who develop tachyphylaxis may produce specific antibodies. However, because it is impossible to identify these antibodies in every patient and the antibody type cannot usually be isolated as a result of morphological variety, it was deemed more appropriate to evaluate tachyphylaxis cases clinically rather than via laboratory testing. Further, it is quite challenging to plan a prospective selection process that involves tachyphylaxis cases because although the development of tachyphylaxis can be observed over time, we cannot predict which patients will develop this condition. Tachyphylaxis may also develop after subsequent doses of ranibizumab, rather than after the first dose. However, patients in whom tachyphylaxis developed later in the treatment course were not included in our study because such cases might not be accurately differentiated from recurrence or tolerance.
In conclusion, our study revealed that intravitreal aflibercept treatment yielded a statistically significant decrease in the short-term CST in eyes that were non-responsive to ranibizumab, as well as in eyes that had developed tachyphylaxis to ranibizumab. However, although positive results were observed in both groups, no statistically significant increase in visual acuity was achieved. The presence of subfoveal PED in eyes with ranibizumab tachyphylaxis decreased significantly after intravitreal aflibercept treatment. Studies with longer durations are needed to determine whether this positive effect of intravitreal aflibercept injection on tachyphylaxis and full resistance would be maintained in the long-term.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

