INTRODUCTION
Intraocular inflammatory diseases are a significant cause of visual impairment and were responsible for 10% of new cases of blindness in the United States in 1990(1). In a large retrospective case series from 1996 of 582 patients with uveitis, 18% went blind in one eye(2). Therefore, adequate treatment of inflammatory eye diseases is important for preserving vision. Uveitis can be classified as infectious or noninfectious. In a survey at Uveitis Service, Hospital das Clinicas, University of São Paulo School of Medicine (HC-FMUSP) in 2004, 37% of all cases of uveitis were noninfectious(3). The current preferred treatment for noninfectious uveitis is based on systemic corticosteroids with or without immunosuppressants or immunomodulators(4,5). More recently, biological agents have been introduced for the treatment of selected cases of noninfectious uveitis(5,6).
The immunosuppressive activity of mycophenolate mofetil (MMF) is based on inhibition of the de novo pathway of purine synthesis by mycophenolic acid (MPA), preventing the replication of T and B lymphocytes(7-9). MMF is effective in the prevention of allograft rejection(7,8) and the treatment of autoimmune diseases(10-13). It inhibits uveitis in animal models(14). In 1998, Kilmartin et al. first reported successful use of MMF as a rescue therapy in nine patients with refractory uveitis(15). Altogether, three prospective case series studies, including 30 patients, have shown that MMF is an efficient immunosuppressant to treat refractory noninfectious ocular inflammation and choroid neovascularization(11,12,16). Serious side effects have been described with doses higher than 3 g per day, e.g., leucopenia, lymphoma, non-melanotic skin cancer, and infections (cytomegalovirus and herpes simplex). At lower doses, the most common side effects are benign, such as diarrhea (in up to 31% of patients)(7,8). In uveitis, the dose is usually up to 2 g per day, and side effects are mild and transient. Plasma concentration monitoring of MPA may optimize the use of MMF and minimize its side effects(17).
The present study aimed to further characterize the efficacy of MMF to control refractory noninfectious uveitis in a tertiary Uveitis Service in São Paulo, Brazil.
METHODS
Patients with noninfectious uveitis followed at the Uveitis Service, Hospital das Clinicas, Faculdade de Medicina, Universidade de São Paulo (HC-FMUSP) from 2007 to 2014 and treated with oral MMF for a minimum of 6 months were retrospectively studied. All patients were being treated with at least one other immunosuppressant when MMF was begun. After reaching an optimal MMF dose, patients were evaluated after 6 (T6), 12 (T12), and 24 months (T24). The optimal MMF dose was defined as ≤3 g/day and/or when primary outcomes were achieved and sustained for at least 6 months. The optimal dose varied for each patient (average 2.2 g/day, range 1.0-3.0 g/day). This study was approved by the Ethics Commission for Analysis of Research Projects of HC-FMUSP (CapPesq 0621/11).
Data collection
Patients on MMF therapy were identified from the Uveitis Service, HC-FMUSP database. The following data were collected: age, gender, uveitis characteristics (anatomical diagnosis, duration, previous systemic therapy), and drug efficacy, and adverse effects. Ocular examination at baseline and at follow-up visits included best-corrected visual acuity measured with a Snellen chart, applanation tonometry, and indirect binocular ophthalmoscopy.
Drug efficacy and tolerance
Primary outcomes were defined as achieving complete control of inflammation in both eyes and/or oral prednisone dose reduction to ≤10 mg per day on two consecutive visits at least 28 days apart. Secondary outcomes evaluated were the time required to reduce oral prednisone to ≤10 mg per day, partial control of ocular inflammation, concomitant use of other immunosuppressants, and side effects or MMF discontinuation. Ocular inflammation (i.e., anterior chamber cells, clinical and angiographic macular edema, and retinal vasculitis) was evaluated according to the Standardization of Uveitis Nomenclature proposed by International Uveitis Society(18).
Statistical analysis
Clinical and epidemiological data were analyzed based on patient characteristics and tabulated as proportions of the study population. Visual acuity measured with a Snellen chart was converted to logMar(16,17). Events are presented as the incidence rate (person-time rate) during the 24 months of observation. The achievement of the primary outcome of oral prednisone dose reduction to ≤10 mg/day occurring at any given time after MMF was started is presented as survival curves, created using the Kaplan-Meier product-limit method. All statistical analyses were calculated using GraphPad Prism (version 6.01 for Windows; GraphPad Software, San Diego, California, USA).
RESULTS
Characteristics of patients included
Sixteen patients (9 male and 7 female), with a mean age of 41 years (range 14 to 57 years), were included in the study (Table 1). Fifteen patients (94%) had intermediate or posterior/diffuse uveitis. Vogt-Koyanagi-Harada and Behçet diseases were the most frequent etiologies (9 patients, 56%). All patients had bilateral uveitis.
Table 1 Clinical characteristics of patients with noninfectious uveitis treated with mycophenolate mofetil
| Number of patients | 16 | |
| Median age (years) at the beginning of treatment (range) | 41 | (14-57) |
| Gender, n (%) | ||
| Male | 9 | (56) |
| Female | 7 | (44) |
| Uveitis classification, n (%) | ||
| Anterior | 1 | ( 6) |
| Intermediate | 3 | (19) |
| Posterior | 3 | (19) |
| Diffuse | 9 | (56) |
| Laterality | ||
| Bilateral | 16 | (100) |
| Median disease duration (years) at start of treatment (range) | 4.1 | (1.3-6.5) |
| Etiology, n (%) | ||
| Vogt-Koyanagi-Harada disease | 5 | (31%) |
| Behçet disease | 4 | (25%) |
| Idiopathatic retinal vasculitis | 3 | (18%) |
| Intermediate uveitis | 3 | (18%) |
| Ankylosing spondylitis | 1 | (6.2%) |
Previous immunosuppressive treatment
The duration of and agents for previous systemic immunosuppressive therapy in each patient is shown in table 2. Three patients (18.7%) had previously received one other immunosuppressive drug (cyclosporine A or azathioprine), seven (43%) had received two (cyclosporine A and azathioprine), and six (37.5%) had received three or more drugs, amongst which were methotrexate, alkylating agents (chlorambucil, cyclophosphamide), and an anti-tumor necrosis factor α agent (infliximab, adalimumab, or etanercept). Twelve patients were on >10 mg/day of oral prednisone, with a mean dose at baseline of 25 mg/day (range 15-60 mg/day). Four patients did not receive prednisone.
Table 2 Immunosuppressant drugs used prior to mycophenolate mofetil in patients with noninfectious uveitis
| Case | PRED | CSA | CLB | CFM | AZA | MTX | Etcept | Infmab | Admab | n |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ● | ● | ● | ● | 3 | |||||
| 2 | ● | ● | ● | ● | ● | 4 | ||||
| 3 | ● | ● | ● | 2 | ||||||
| 4 | ● | ● | ● | 2 | ||||||
| 5 | ● | ● | ● | 3 | ||||||
| 6 | ● | ● | ● | 2 | ||||||
| 7 | ● | ● | ● | ● | 3 | |||||
| 8 | ● | ● | ● | ● | ● | ● | 5 | |||
| 9 | ● | ● | 2 | |||||||
| 10 | ● | ● | ● | ● | 3 | |||||
| 11 | ● | 1 | ||||||||
| 12 | ● | 1 | ||||||||
| 13 | ● | ● | ● | 2 | ||||||
| 14 | ● | ● | 1 | |||||||
| 15 | ● | ● | ● | 2 | ||||||
| 16 | ● | ● | ● | 2 | ||||||
| N (%) | 12 (75) | 13 (81) | 3 (18) | 2 (12.5) | 13 (81) | 2 (12.5) | 1 (6.2) | 2 (12.5) | 2 (12.5) |
PRED= prednisone; CSA= cyclosporine A; CLB= chlorambucil; CFM= cyclophosphamide; AZA= azathioprine; MTX= methotrexate; Etcept= etarnercept; Infmab= infliximab; Admab= adalimumab.
MMF treatment start and its efficacy
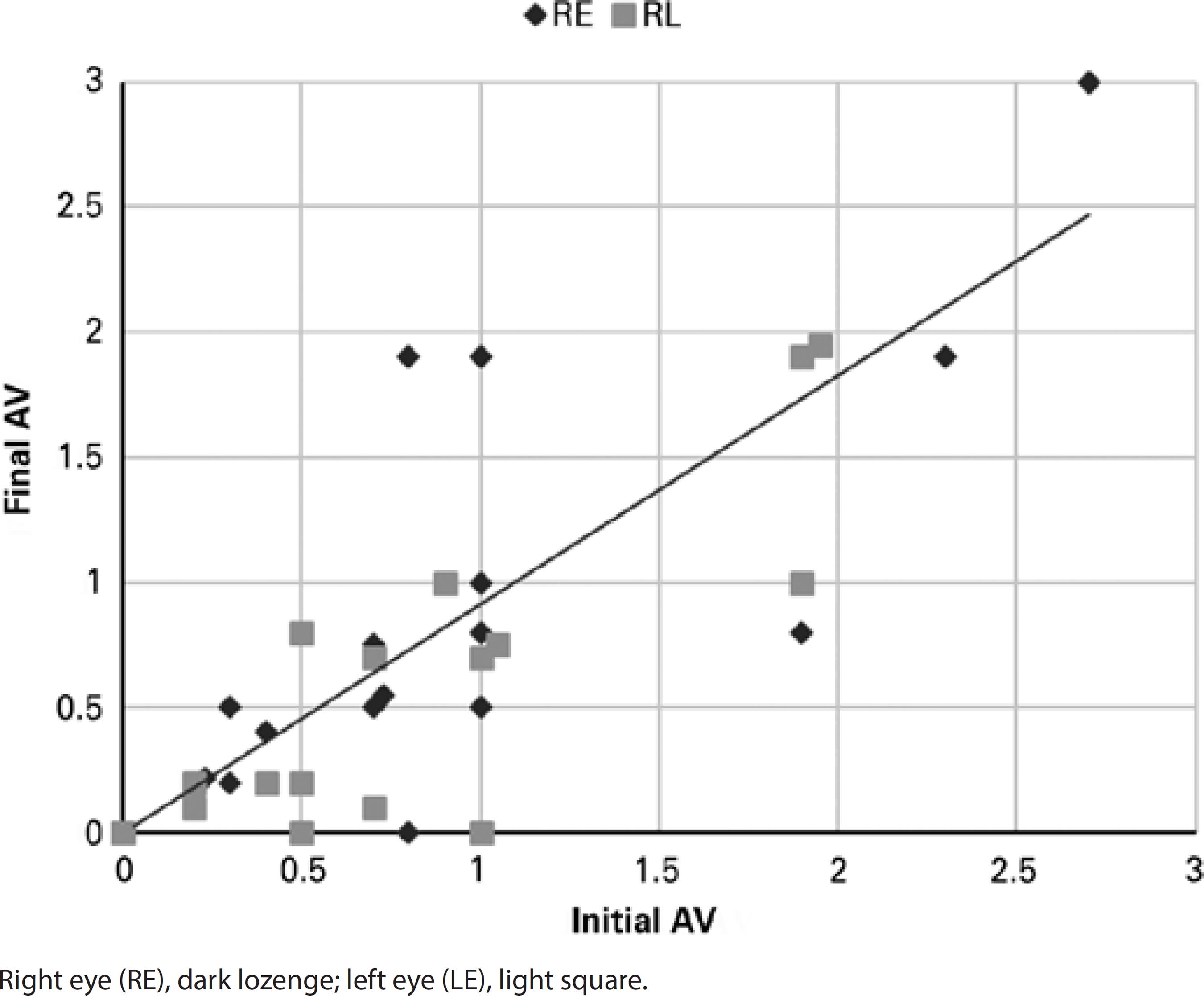
The mean interval from beginning MMF until the optimal dose was achieved was 1.2 years (range 0.5-2.6 years). At T24, 10 out of 12 patients (83%) who had required >10 mg per day of prednisone at T0 were able to reduce the dose to ≤10 mg per day (Figure 1). There was an increase in complete and partial inflammation control rates at T12 as compared with T6 (Table 3). Among the 6 patients with a longer follow-up, 2 (33%) had disease remission after 60 and 40 months of MMF treatment. Functional improvement, measured by an increase in or stabilization of visual acuity, was observed in 12 patients (75%) after 24 months of MMF (Figure 2).

Figure 1 Reduction of prednisone to ≤10 mg/day in patients with noninfectious uveitis under mycophenolate mofetil therapy.
Table 3 Efficacy of mycophenolate mofetil in patients with noninfectious uveitis after 6 (T6), 12 (T12), and 24 (T24) months of treatment
| Efficacy | T6, n (%) | T12, n (%) | T24, n (%) |
|---|---|---|---|
| Prednisone ≤10 mg/day* | 7 (58.0) | 8 (67.0) | 10 (83.0) |
| Inflammation control | |||
| Complete | 2 (12.5) | 5 (31.2) | 5 (31.2) |
| Partial | 1 ( 6.2) | 2 (12.5) | 2 (12.5) |
*data for the 12 patients at T0 who were on prednisone >10 mg/day.
Side effects
Gastrointestinal disturbances were the most frequent complaint requiring MMF dose decrease or discontinuation (in 1 patient) (Table 4). One patient, with a follow-up of 58 months, developed sepsis 51 months after achieving an optimal MMF dose (3 g/d). This patient had favorable outcome after appropriate antibiotic therapy.
DISCUSSION
In this retrospective case series, MMF was effective in treating refractory noninfectious uveitis.
Most previous studies of MMF in noninfectious uveitis are retrospective. In the five published prospective studies, which included a total of 85 patients, 47% had better control of inflammation, and 100% were able to reduce the prednisone dose to ≤10 mg per day. Best-corrected visual acuity improved or stabilized in 100%, achieving >20/40 in 74% of patients(11,12,16,19-21). A relevant observation of our present study is that the longer MMF is used, the better the results. Doycheva and Zierhut reported an estimated 94% to 96% control of inflammation with a prednisone dose of ≤10 mg per day after 1 or 2 years of MMF treatment(22). Teoh found an 85% probability of reducing prednisone to ≤10 mg per day after one year of treatment(23). In our case series, 67% of patients reached this ideal prednisone dose after 1, and 83% of patients reached it after 2 years of MMF treatment. It is of note that, at the final evaluation, three patients were not taking any other immunosuppressants, and disease remission was observed in two. Addition of another immunosuppressant during therapy with MMF was not needed in any patient.
The average MMF dose in our study was 2.25 g/day (range 1-3 g/d), similar to other studies(20,22,24). Most of the side effects in our series were observed with a dose of 3 g per day (three out of four patients). Doses ≥3 g/day may increase the risk of toxicity(4-6). The appropriate dose may lie between 2 and 3 g/day and may require individualization depending on the clinical course or other factors, e.g., monitoring of MMF plasma concentrations(17).
MMF was moderately well tolerated, with four patients (25%) experiencing side effects. Among these was one patient with a severe side effect, i.e., sepsis, after 58 months on MMF. Gastrointestinal disturbances are indeed the most frequently described side effect in the literature (23%-35% versus 21% in the present study). The formulation of mycophenolate sodium may improve gastrointestinal symptoms(24). The incidence of infection varies more widely in the literature (10% to 46% versus 14% in the present study)(4,20,23). Therefore, as reported in the literature, MMF is a well-tolerated immunosuppressant with a risk of adverse events similar to methotrexate(20). It is important to note that white blood count cells and liver function should be monitored every three months(4-6).
In conclusion, this small retrospective case series is consistent with what is reported in the literature concerning the efficacy and tolerability of MMF in noninfectious uveitis. It may take treatment for at least one year to determine the efficacy of MMF.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

