INTRODUCTION
Retinal vascular occlusive disorders are the second most common cause of vision loss among patients with retinal vascular disease(1). Macular edema (ME) is a major complication of vein occlusion and the predominant cause of acute vision loss(2). Many factors such as increased hydrostatic pressure in the venous circulation, inflammation, endothelial damage, and increased vascular permeability contribute to the development of ME(3). Grid laser photocoagulation(1), antivascular endothelial growth factors (VEGFs)(4-6), and triamcinolone injection(7) have demonstrated efficacy in the treatment of ME caused by retinal vein occlusion (RVO). These treatments aim to reduce existing damage to retinal cells, particularly in the photoreceptor layer, and provide visual improvement by reducing edema, ischemia, and cellular damage. Recently, the intravitreal dexamethasone implant, Ozurdex, has been used as a new treatment modality to reduce ME and increase visual acuity in patients with both types of RVO: branch retinal vein occlusion (BRVO) and central retinal vein occlusion (CRVO)(8).
Clinical assessment of the choroid is typically conducted by indocyanine green angiography (ICGA) or B-scan ultrasonography; however, neither method allows for accurate cross-sectional imaging. Recently, a new approach to optical coherence tomography (OCT), known as enhanced depth imaging (EDI)-OCT, has demonstrated utility in imaging the full thickness of the choroid in a noninvasive, rapid, objective, and reliable manner(9). Choroidal thickness can be affected by ocular pathologies such as age-related macular degeneration(10), central serous chorioretinopathy(11), Vogt-Koyanagi-Harada disease(12), macular holes(13), high myopia(14), and polypoidal choroidal vasculopathy(15), in addition to systemic diseases such as diabetes. Several previous studies have evaluated choroidal thickness in RVO patients(16-18). However, the results of these studies were contradictory. Du et al.(16) reported no difference in subfoveal choroidal thickness (SFCT) between eyes with longstanding BRVO and normal contralateral eyes. In contrast, Lee et al.(17) reported greater choroidal thickness in eyes with BRVO and CRVO compared with normal contralateral eyes. To date, no studies have examined choroidal thickness in treatment-naïve eyes with BRVO before and after Ozurdex injection.
In the present study, EDI-OCT was used to investigate the short-term effects of Ozurdex injection on choroidal thickness in patients with BRVO by comparing choroidal thickness with the eyes of age- and gender-matched healthy control and normal contralateral eyes.
METHODS
Study population and design
This prospective comparative study was performed in the Departments of Ophthalmology at Kayseri Education and Research Hospital. The present study followed the tenets of the Declaration of Helsinki and was approved by the local ethics committee. All participants received both oral and written information about the study, and each participant provided written informed consent. Participants were recruited into two groups: the study group consisting of 39 patients with BRVO and the control group consisting of 35 healthy volunteers. Inclusion criteria were as follows: recent-onset (<4 weeks) and treatment-naive unilateral BRVO with ME. The diagnosis of BRVO was made according to the results of clinical examination and supported by fundus fluorescein angiography and OCT measurements.
Exclusion criteria
Ocular exclusion criteria for the present study were as follows: prior history of significant ocular disease, refractive error of either less than -3 D or more than +3 D, amblyopia, intraocular pressure (IOP) readings greater than 21 mmHg, glaucoma, history of uveitis, retinal disease (except for BRVO), ocular trauma or tumor, poor image quality, and dense media opacities. Patients with a history of previous intraocular laser therapy or the use of any intravitreal injections were also excluded from the present study.
Examination protocol and study measurements
All participants in both groups underwent a complete examination that included Snellen best-corrected visual acuity, biomicroscopy, IOP measured by Goldmann applanation tonometry, and dilated fundus examination with axial length (AL) and OCT measurements. AL measurements were measured with the IOL Master 500 (Carl Zeiss Meditec Inc, Jena, Germany). OCT measurements were retaken 1 month after intravitreal dexamethasone implant injection in the study group. Due to diurnal fluctuations, all examinations were performed between 9 am and 11 am.
OCT measurements
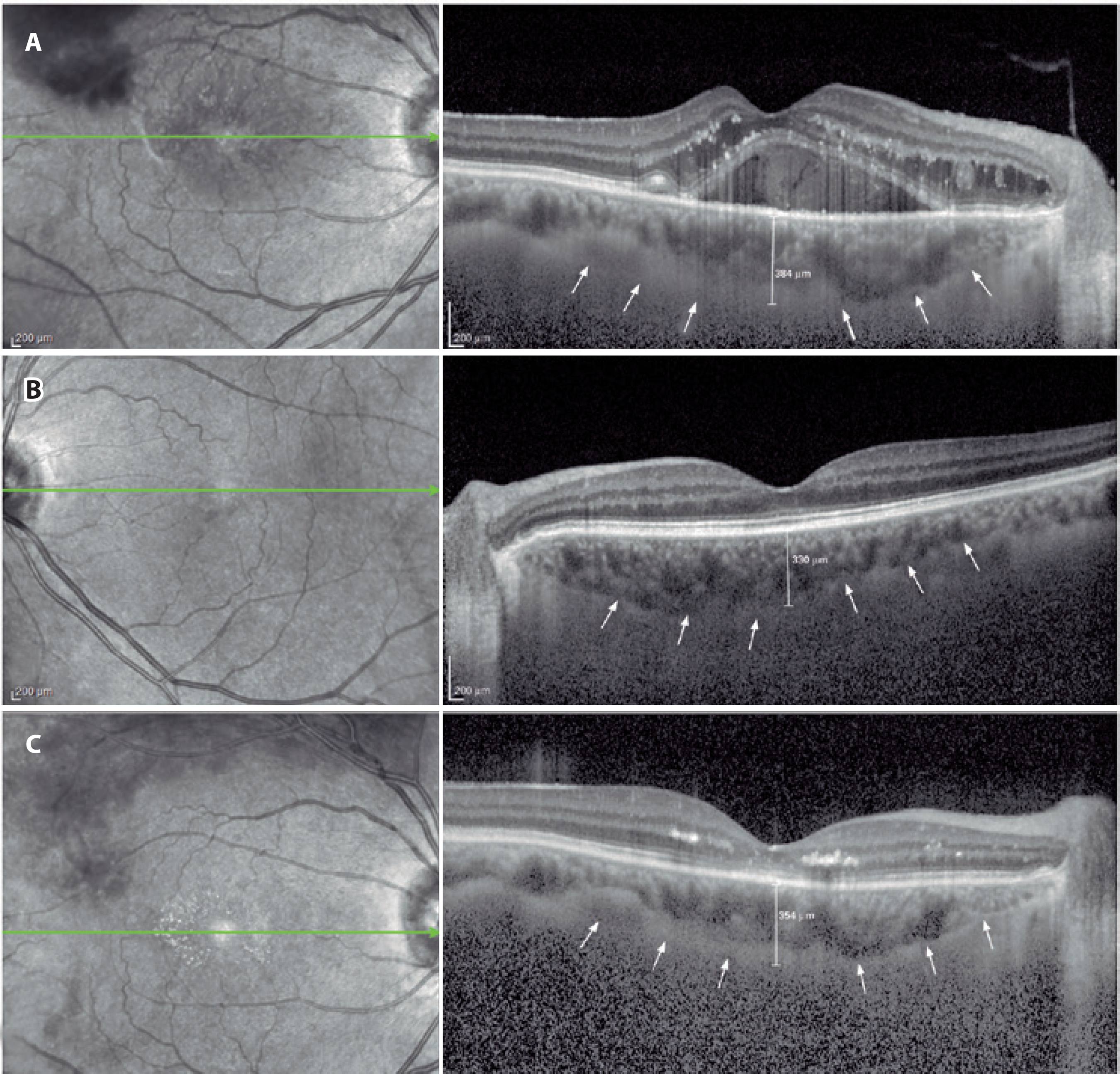
Following detailed ophthalmologic examinations, a third-generation Spectralis OCT device (software version 5.6.3.0; Spectralis OCT, Heidelberg Engineering, Dossenheim, Germany) was used for ocular assessments. The method of obtaining EDI-OCT images has been previously reported(9,19). SFCT was determined as the vertical distance from the hyperreflective line of the hyperreflective retinal pigment epithelium to the line of the inner surface of the sclera centered on the fovea, which was taken using a tool with built-in linear measuring. A representative EDI-OCT choroidal image is presented in figure 1. Images were captured by one experienced clinician and assessed by another experienced clinician. Group identities remained anonymous to both clinicians.
Statistical analysis
All statistical tests were performed using SPSS (Statistical Package for the Social Sciences) version 20. In the control group, OCT measurements from the right eye were used for analyses. For each continuous variable, normality was checked using the Kolmogorov Smirnov test. Differences in categorical variables between groups were analyzed using the χ2 test. An independent t-test was used to compare variables between study and control groups. The paired t-test was used for comparisons between BRVO eyes and fellow eyes. The paired t-test was also used for comparisons of preinjection and postinjection measurements. p-Values less than 0.05 were considered statistically significant.
RESULTS
Table 1 shows the demographic characteristics of the study and control groups. No statistically significant difference in sex or age was observed between the two groups (p=0.929 and p=0.432, respectively). Table 2 shows the results of IOP, AL, and SFCT measurements in the control and study groups. Mean SFCT measurements in eyes with BRVO differed significantly from healthy control eyes and fellow eyes (p=0.044 and p=0.009, respectively). However, no statistically significant differences in IOP or AL values were observed between the study and control groups (p>0.05). Table 3 shows the comparison of choroidal thickness between preinjection and postinjection periods. A statistically significant difference in mean SFCT measurements was observed at 1 month after Ozurdex injection in eyes with BRVO (p=0.004).
Table 1 Patient demographics and characteristics
| Study group | Control group | p-value | |
|---|---|---|---|
| Number of eyes/patients | 39/39 | 35/35 | - |
| Sex | |||
| Female | 23 (59%) | 21 (60%) | 0.929* |
| Male | 16 (41%) | 14 (40%) | |
| Age (years) | |||
| Mean ± SD | 64.71 ± 10.99 | 62.91 ± 8.24 | 0.432** |
| Range | 41-89 | 46-83 |
*= Chi-Square test;
**= independent-sample t test.
Table 2 Comparison of choroidal thickness and other clinical measurements between study group and control group
| Study group | p-values | |||||
|---|---|---|---|---|---|---|
| Eyes with BRVO | Fellow eyes | Control group | BRVO versus control* | BRVO versus fellow** | Fellow versus control* | |
| SFCT (µm) | ||||||
| Mean ± SD | 299.41 ± 55.86 | 283.76 ± 57.44 | 276.14 ± 39.06 | 0.044 | 0.009 | 0.511 |
| Range | 188-425 | 164-390 | 190-378 | |||
| AL (mm) | ||||||
| Mean ± SD | 22.82 ± 0.64 | 23.04 ± 0.55 | 22.98 ± 0.69 | 0.307 | 0.112 | 0.667 |
| Range | 21.24-23.98 | 21.98-24.11 | 21.87-24.02 | |||
| IOP (mmHg) | ||||||
| Mean ± SD | 14.69 ± 2.86 | 14.89 ± 2.41 | 15.42 ± 2.42 | 0.240 | 0.570 | 0.349 |
| Range | 9-20 | 10-20 | 10-21 | |||
AL= axial length; BRVO= branch retinal vein occlusion; IOP= intraocular pressure; SD= standard deviation; SFCT= subfoveal choroidal thickness.
*= independent-sample t-test;
**= paired-t test.
Table 3 Comprasion of choroidal thickness between preinjection and postinjection
| Preinjection | 1 month postinjection | p-value* | |
|---|---|---|---|
| SFCT (µm) | |||
| Mean ± SD | 299.41 ± 55.86 | 279.64 ± 50.96 | 0.004 |
| Range | 188-425 | 169-396 |
SD= standard deviation; SFCT= subfoveal choroidal thickness.
*= Paired-t test.
DISCUSSION
The present study demonstrated that SFCT in recent-onset (<4 weeks) treatment-naive BRVO eyes with ME was greater than that in unaffected contralateral and control eyes. Furthermore, choroidal thickness decreased in response to intravitreal dexamethasone implant treatment.
RVO is the second most common vascular disease leading to decreased visual acuity, with an annual prevalence 4.42 in 1,000 persons(20), slightly lower than diabetic retinopathy. Kolar(21) conducted a meta-analysis to detect risk factors of RVO, identifying advancing age. Other reported risk factors included systemic conditions such as hypertension, arteriosclerosis, diabetes mellitus, hyperlipidemia, vascular cerebral stroke, blood hyperviscosity, and thrombophilia.
Treatment options for ME after the occurrence of BRVO include laser photocoagulation, intravitreal VEGF antagonists, and intravitreal corticosteroids(1,4-8).
The dexamethasone 0.7-mg intravitreal implant (Ozurdex®, Allergan, Irvine, CA, USA) is a sustained-biodegradable implant containing the corticosteroid, dexamethasone. Corticosteroids including dexamethasone are known to have anti-inflammatory and antiangiogenic properties and may inhibit the expression of VEGF and other proinflammatory cytokines such as IL-6, ICAM-1, and MCP-1(8,22,23).
The choroid is the vascular layer between the retina and the sclera that provides blood supply to the eye and plays an important role in ocular nutrition.
Choroidal blood flow is the highest of any tissue in the body and is necessary to satisfy the normal metabolic demands of the outer retina. A structurally and functionally normal choroidal vasculature is essential for retinal function(24), and the choroid itself is important for visual acuity. Eyes with a relatively thicker choroid at baseline may have greater choroidal blood supply and choriocapillaris, which may increase the possibility of a full recovery from ME(17).
Tsuiki et al.(18) posited that VEGF may increase vascular permeability and induce fenestrations of the choriocapillaris, which may in turn increase choroidal thickness. In eyes with RVO, VEGF expression is increased in retinal endothelial cells, pericytes, RPE, Müller cells, ganglion cells, and astrocytes as a result of hypoxia. Choroidal thickness is also mediated by vascular dilatation induced by nitric oxide (NO) production in response to VEGF expression. Choroidal thickness may increase as a result of either vasodilataton or edema. The findings of the present study support the hypothesis of Tsuiki et al.
Since Spaide et al.(9) developed a method called EDI-OCT, which enables both in vivo cross-sectional imaging of the choroid and the measurement of choroidal thickness, an increasing number of studies have reported the choroidal thickness in eyes with various diseases(10-18).
In a population-based cross-sectional study, Du et al.(16) reported no difference in SFCT between eyes with RVO and normal contralateral eyes. In contrast, Tsuki et al.(18) reported significantly greater SFCT in CRVO eyes compared with normal contralateral eyes. In addition, Lee et al.(17) recently reported significantly greater SFCT in eyes with CRVO and BRVO compared with normal contralateral eyes. These inconsistencies may be attributable to differences in patient characteristics. In contrast to the present study, Du et al. included subjects with longstanding RVOs without marked cystoid ME. The present findings were similar to the findings of Tsuki and Lee's studies. The present study included recent onset (<4 weeks) and treatment-naïve BRVO patients, which allowed the evaluation of early choroidal changes in BRVO without any effect of treatment modalities such as anti-VEGF agents or laser treatments.
Tsuiki et al.(18) reported decreased SFCT 1 month after intravitreal bevacizumab treatment in patients with CRVO. Lee et al.(17) also reported that increased SFCT in CRVO and BRVO patients decreased after injection of the dexamethasone implant. The present study found that the effects of the dexamethasone implant on choroidal thickness were similar to those reported by previous studies. However, the present study assessed only the effects of Ozurdex on choroidal thickness in patients with recent-onset BRVO.
Corticosteroids inhibit the synthesis of NO and decrease tissue edema. Studies have shown that corticosteroids downregulate VEGF expression and other pro-inflammatory cytokines(22,25). Accordingly, the dexamethasone implant may reduce choroidal thickness via this mechanism.
The present study had several limitations. First, choroidal vessels were not evaluated by ICGA. If ICGA had been performed, it may have been possible to compare angiographic changes on OCT. Second, the present study sample size was somewhat small, and the follow-up period was relatively short, both of which decrease statistical generalizability. Third, SFCT measurements were obtained manually. Automated software may allow more objective evaluation by erasing any potential bias. Future studies with larger numbers of subjects, longer follow-up durations, and the use of automated software are required to fully validate the results of the present study.
In conclusion, SFCT in recent-onset, treatment naïve BRVO eyes is significantly greater in both normal contralateral eyes and age- and sex-matched healthy control eyes. Choroidal thickness is significantly decreased 1 month after the injection of the dexamethasone implant. SFCT may have utility in assessing the effects of antiedematous treatment on choroidal vascular structures by measuring choroidal thickness noninvasively with EDI OCT, thereby allowing patients to be observed more reliably. Further studies are required to elucidate the role of the choroid in the pathogenesis of BRVO.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

