INTRODUCTION
The Valsalva maneuver (VM) is performed frequently in daily activities. It causes various transient physiological changes, including elevated blood pressure, increased intrathoracic pressure, increased peripheral venous pressure stimulation of the peripheral sympathetic system, and increased intraocular pressure (IOP)(1,2). The mechanism for the transient increase in IOP during VM has not been completely explained. One hypothesis is that the increased systemic venous pressure is transmitted through the jugular, orbital, and vortex veins to the choroid, causing vascular engorgement and increases in choroidal volume and IOP. Another hypothesis relates this IOP increase to an increase in episcleral venous pressure(1-4).
The pathophysiology of glaucoma is likely multifactorial, with IOP-dependent and vascular factors involved. Patients with primary open-angle glaucoma (POAG) have been shown to have vascular dysregulation that leads to either local vasospasm or impaired autoregulation(5,6). Researchers have showed that VM causes significant changes in anterior chamber parameters and an increase in uveal thickness that leads to a transient increase in IOP in healthy subjects(3,4). These dynamic changes may also induce acute angle-closure glaucoma (AACG)(7,8). Moreover, normotensive glaucoma (NTG) has been reported to be more common in patients with exposure to increases in IOP caused by intrathoracic and intra-abdominal pressure, such as that from playing wind instruments, lifting weights regularly, asthma, or having chronic urinary tract or intestinal obstruction(9).
We hypothesized that VM may facilitate glaucomatous optic neuropathy (GON). Based on this theory, we designed this study to investigate the acute changes in IOP, optic disc morphology, choroidal thickness, and anterior chamber parameters during VM in healthy subjects. To the best of our knowledge, this article is the first report on the acute effects of VM on peripapillary choroidal thickness and the second report on the acute effects of VM on optic disc morphology.
METHODS
Sixty eyes of 60 healthy subjects were included in this prospective observational study. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Gaziantep University Clinical Research Ethics Committee. Detailed informed consent was obtained from each participant.
Subjects
Healthy subjects who had refractive errors between -1.00 and +1.00 diopters (D) spherical equivalent were accepted in this study. One eye of each subject was randomly chosen. Volunteers who could not open their eyelid wide, were physically disabled to perform VM, had poor ocular fixation, had a history of ocular surgery or ocular lens wear, used systemic or ocular medications, or had a family history of glaucoma and systemic or ocular diseases were excluded. All participants underwent a complete ophthalmic examination, including visual acuity testing, slit-lamp biomicroscopy, and fundus examination. Only participants with an IOP ≤21 mmHg and normal optic nerve head appearance [without peripapillary atrophy, glaucomatous cupping, tilted disc, or retinal nerve fiber layer (RNFL) loss] were included in the study.
Measurement technique and instruments
VM was performed with expiratory pressure ranging between 35 and 40 mmHg by blowing through the mouthpiece attached to a manometer for 10 s in a sitting position for each measurement. All measurements were performed by experienced physicians. First, a resting measurement was taken 1 min before VM, and measurements during VM were taken 10 s after 35-40 mmHg pressure was achieved(1,3,4). The following measurements were taken 15 min after each VM.
The central corneal thickness (CCT), anterior chamber depth (ACD; distance from the corneal endothelium to the anterior lens capsule), anterior chamber volume (ACV), anterior chamber angle (ACA), and pupil diameter (PD) measurements were performed by the same physician using Sirius® Scheimpflug topography (Costruzione Strumenti Oftalmici, Florence, Italy), which combines a monochromatic 360º rotating Scheimpflug camera and a Placido's disc to analyze the anterior segment by obtaining 25 radial sections of the cornea and anterior chamber. Three repeated measurements were taken consecutively and averaged before and during VM for analysis. Subsequently, five measurements of axial length (AL) were taken using a LENSTAR LS 900® optical low-coherence reflectometry biometer (Haag Streit AG, Koeniz, Switzerland) and averaged. The biometer was calibrated daily before the measurements were taken. The participants were asked to blink immediately before the measurements to create an optically smooth tear film over the cornea.
The subfoveal choroidal thickness and peripapillary choroidal thickness measurements were performed using Heidelberg® Spectralis® (Heidelberg Engineering, Heidelberg, Germany) spectral-domain optical coherence tomography (SD-OCT) with the enhanced depth imaging (EDI) modality. For each subject, the scan pattern used on the Spectralis® was a line scan of 30º consisting of 768 A-scans per frame. Horizontal EDI B-scans averaged 100 times with the aid of eye tracking and were centered on the fovea before and during VM. Subsequently, a 360º 3.4-mm diameter peripapillary circle scan was performed using the default glaucoma application and the preset circular RNFL scan, which consisted of 100 averaged EDI B-scans obtained before and during VM. These scans were marked as the subject's baseline and were used for referencing the subsequent scan using the "follow-up" function of the Spectralis® Heidelberg®, allowing the scans to be performed in the same position during VM.
The subfoveal choroidal thickness was measured from the outer limit of the retinal pigment epithelium (RPE) to the choroidal-scleral junction at the center of the fovea. Subsequently, peripapillary choroidal thickness was measured from the outer limit of the RPE and the choroidal-scleral junction. The measurements obtained included the mean peripapillary choroidal thickness, and the mean choroidal thickness of the temporal, superior temporal, superior, superior nasal, nasal, inferior nasal, inferior, and inferior temporal segments using Heidelberg Eye Explorer software (Heidelberg Engineering). The measurements of choroidal thickness were performed independently by two clinicians who were masked in terms of the groups and measurement time. Subsequently, the mean values for each point were recorded. Differences in measurement between the interpreters larger than 10% were excluded from the study.
The optic disc parameters were measured by the same physician using the RTVue® SD-OCT (Model RT 100; Optovue Inc., Fremont, CA, USA). The RTVue uses a scanning laser diode with a wavelength of 840 ± 10 nm to provide images of optic nerve head microstructures. Optic disc parameters were then measured with the optic nerve head scan protocol (software version 6.3.2.73), using 12 radial line scans 3.4 mm in length (452 axial scans per line). This version of the software automatically draws the contour line of the disc margin by estimating the RPE edges and then generates optic disc parameters. The integrated signal strength index (SSI) was used to control for image quality. SSI measurements of 50 and above were accepted; scans showing movement or decentration artifacts were repeated. The optic disc area, rim area, cup area, cup-to-disc area ratio, horizontal cup-to-disc ratio, vertical cup-to-disc ratio, rim volume, cup volume, and nerve head volume values were obtained from the RTVue-100® scans before and during VM.
IOP was measured by the same physician using a Tono-Pen® XL (TPXL; Medtronic Solan, Jacksonville, FL, USA). After the topical anesthesia is administered, the operator touches the covered tip of the TPXL to the cornea. Four individual measurements were taken by slightly touching the central cornea before and during VM. When four valid readings with a variance of 5% were obtained, the mean IOP was noted.
Statistical analysis
SPSS 16.0 software for Windows (SPSS Inc., Chicago, IL, USA) was used to analyze the outcomes. All data were presented as the mean ± standard deviation. We used the paired samples t- test to compare the values obtained at baseline and during VM, and multiple regression analysis was performed for detecting possible relationships between the anterior chamber parameters and IOP; p <0.05 was regarded as statistically significant.
RESULTS
Sixty eyes of 60 healthy volunteers were examined. The subjects were aged 20-51 years, and their mean age was 34.7 ± 8.8 years. There were 32 men (53.3%) and 28 women (46.7%). Thirty right eyes (50%) and 30 left eyes (50%) were included in the analysis. The effects of VM on IOP, AL, CCT, ACD, ACA, ACV, and PD are shown in table 1.
Table 1 Intraocular pressure, axial length, central corneal thickness, anterior chamber depth, anterior chamber angle, anterior chamber volume, and pupillary diameter measurements in a resting position and during the Valsalva maneuver
| Resting position | Valsalva maneuver | ||
|---|---|---|---|
| Parameters | mean ± SD | mean ± SD | p-value |
| IOP, mmHg | 15.70 ± 1.50 | 20.40 ± 2.60 | <0.001 |
| Range, mmHg | (13-19) | (16-25) | |
| AL, mm | 23.65 ± 0.87 | 23.63 ± 0.91 | 0.209 |
| CCT, µm | 538.50 ± 3.20 | 532.90 ± 3.10 | <0.001 |
| ACD, mm | 3.11 ± 0.04 | 3.06 ± 0.04 | <0.001 |
| ACA, degree | 41.20 ± 5.80 | 39.50 ± 6.10 | <0.001 |
| ACV, mm3 | 160.80 ± 22.10 | 156.70 ± 22.60 | <0.001 |
| PD, mm | 4.48 ± 0.55 | 4.84 ± 0.59 | <0.001 |
IOP= intraocular pressure; AL= axial length; CCT= central corneal thickness; ACD= anterior chamber depth; ACA= anterior chamber angle; ACV= anterior chamber volume; PD= pupil diameter; SD= standard deviation.*Paired samples t-tests were used.
CCT, ACD, ACA, and ACV were significantly decreased during VM. VM did not have any significant influence on the AL values, but it significantly increased the IOP and PD values. Multiple regression analysis was performed to predict IOP from AL, ACD, ACA, ACV, and PD. These variables failed to predict IOP [F (5,54)=0.347, p =0.882, R2=0.031].
The effects of VM on subfoveal and peripapillary choroidal thickness are shown in table 2. VM did not have any significant effects on subfoveal and peripapillary choroidal thickness.
Table 2 Subfoveal and peripapillary choroidal thickness measurements in a resting position and during the Valsalva maneuver
| Resting position | Valsalva maneuver | ||
|---|---|---|---|
| Location | mean ± SD | mean ± SD | p-value |
| Subfoveal choroidal | 341.6 ± 51.9 | 341.6 ± 52.0 | 0.837 |
| thickness | |||
| Temporal (PCT) | 195.4 ± 42.9 | 195.5 ± 43.2 | 0.568 |
| Superotemporal (PCT) | 206.3 ± 46.6 | 206.2 ± 46.3 | 0.435 |
| Superior (PCT) | 220.1 ± 52.4 | 219.6 ± 51.8 | 0.146 |
| Superonasal (PCT) | 224.0 ± 51.0 | 224.2 ± 51.1 | 0.491 |
| Nasal (PCT) | 214.2 ± 48.9 | 214.2 ± 48.6 | 0.891 |
| Inferionasal (PCT) | 203.2 ± 46.3 | 203.0 ± 46.5 | 0.266 |
| Inferior (PCT) | 149.5 ± 45.9 | 149.6 ± 46.0 | 0.840 |
| Inferotemporal (PCT) | 178.7 ± 43.0 | 178.9 ± 43.1 | 0.760 |
| Average (PCT) | 198.5 ± 45.7 | 198.8 ± 46.2 | 0.131 |
All measurements were in micrometers. PCT= peripapillary choroidal thickness; SD= standard deviation.*Paired samples t-tests were used.
The effects of VM on optic disc parameters are shown in table 3. The cup volume decreased and the horizontal cup-to-disc ratio significantly increased during VM. However, VM did not have any significant influence on optic disc area, rim area, cup area, cup-to-disc area ratio, vertical cup-to-disc ratio, rim volume, and nerve head volume.
Table 3 Optic disc parameters in a resting position and during the Valsalva maneuver
| Resting position | Valsalva maneuver | ||
|---|---|---|---|
| Optic disc parameters | mean ± SD | mean ± SD | p-value |
| Optic disc area (mm2) | 1.920 ± 0.330 | 1.920 ± 0.320 | 0.494 |
| Rim area (mm2) | 1.050 ± 0.310 | 1.060 ± 0.340 | 0.587 |
| Cup area (mm2) | 0.870 ± 0.510 | 0.860 ± 0.520 | 0.545 |
| Cup-to-disc area ratio | 0.434 ± 0.192 | 0.430 ± 0.203 | 0.645 |
| Horizontal CDR | 0.732 ± 0.182 | 0.760 ± 0.180 | 0.006 |
| Vertical CDR | 0.670 ± 0.140 | 0.660 ± 0.150 | 0.343 |
| Rim volume (mm3) | 0.133 ± 0.099 | 0.147 ± 0.149 | 0.437 |
| Cup volume (mm3) | 0.251 ± 0.253 | 0.240 ± 0.239 | 0.049 |
| Nerve head volume (mm3) | 0.229 ± 0.134 | 0.246 ± 0.180 | 0.415 |
CDR= cup-to-disc ratio; SD= standard deviation.*Paired sample t-tests were used.
DISCUSSION
Glaucoma is one of the leading causes of blindness worldwide, and its pathophysiology is still not understood. High IOP is one of the most important risk factors for glaucoma(10). It has been shown that IOP increases transiently during daily activities such as weight lifting, playing wind instruments, coughing, or isometric exercises. The IOP rise during VM has been correlated with both the amount of expiratory force produced and the length of time taken in the maneuver(11). Previous studies using ultrasound biomicroscopy have showed that increases in uveal and iris thickness lead to significant recessing and narrowing of the ACA and a transient increase in IOP(12). In our study, we observed that IOP was significantly increased during VM. The IOP rise during VM has been reported to be as low as 2 mmHg and as high as 26 mmHg in different studies(11,13). Our results confirmed the results of previous studies(3,4,9,11). The wide range of IOP difference values in previous studies could be explained by the amount of expiratory force, the length of time taken in the maneuver, or the use of different measurement techniques. Postural changes during VM, structural corneal rigidity, and hypofluorescence of the precorneal tear film may cause errors in the use of Goldmann applanation tonometry (GAT);(14) therefore, we prefer measuring IOP values using TPXL.
We observed that the CCT, ACD, ACA, and ACV parameters were significantly decreased during VM as assessed by Sirius® Scheimpflug topography. These outcomes are consistent with those from previous studies. Pekel et al.(3) performed measurements with the Pentacam system (Oculus, Wetzlar, Germany) and showed that VM decreases CCT, ACD, ACA, and ACV significantly. Mete et al.(4) also reported that VM decreases CCT and ACD significantly using the LENSTAR® optical low-cohorence reflectometry biometer. Researchers have hypothesized that the decrease in CCT may be caused by mechanical stretching of the cornea or thinning of the tear film during VM(3,4). Wang et al.(13) reported that the decrease in ACD might be caused by the forward movement of the iridolenticular diaphragm because of the thickening of the ciliary body and choroid during VM as assessed by ultrasound biomicroscopy. Falcão et al.(2) showed that a VM does not change the choroidal thickness at the posterior pole. We believe that the increase in the volume of the anterior part of the choroid and the forward movement of the iridolenticular diaphragm by the thickening of the ciliary body explains the anterior segment changes during VM. We also observed that PD was significantly increased during VM. The change in PD could possibly be explained by the increased peripheral sympathetic stimulation and the increase in lens thickness, which pushes the iridolenticular diaphragm somewhat anteriorly and thus increases PD. Even this mild dilation could lead to further narrowing of the anterior chamber and iridocorneal angle(1,4). These dynamic changes may induce AACG(7,8). Moreover, NTG has been reported to be more common in patients with exposure to increases in IOP caused by intrathoracic and intra-abdominal pressure(9).
This study showed that VM did not have any significant effect on AL. This finding confirms the results from our previous study(4). Researchers have shown the relationship between changes in IOP and AL; however, the precise mechanism has not yet been identified(15). Temporary changes in AL might be explained by iris thickness or mechanical stretching of the sclera or thinning of choroid thickness during VM.
Two theories have been used to describe the pathogenesis of GON. The mechanical theory is related to insufficient blood supply to the prelaminar optic nerve because of increased IOP(1,16). It is not completely understood how the VM increases the IOP. One explanation might be that the increase in systemic venous pressure causes reduced blood flow in the vortical veins, which in turn increases the choroidal volume and IOP(17). Another explanation might be that an increase in the episcleral venous pressure may increase IOP(18). The vascular theory is related to vascular dysregulation, which leads to either local vasospasm or impaired autoregulation(5,19). Because the branches of the peripapillary choroidal arterioles contribute to the blood supply of the optic nerve head, the peripapillary choroid may play an important role in the cause of glaucoma(19,20). Histopathology studies have showed that choroidal thickness, especially in the peripapillary region, is thinner in glaucomatous than in normal eyes(21,22). Moreover, researchers have reported reduced retrobulbar blood flow, choroidal blood flow, and retinal blood flow using different techniques in patients with OAG(23-25). There are few studies on the effects of VM on the choroid. Falcão et al.(2) reported that VM did not have any significant influence on the macular choroidal thickness, and IOP changes that occur during VM are not related to volume changes of the posterior choroid. Other mechanisms such as increases in anterior choroidal thickness or episcleral venous pressure are probably more related to this phenomenon. In the present study, we investigated peripapillary choroidal thickness during a VM in a different manner from that in previous reports. To the best of our knowledge, the present article is the first report on the acute effects of VM on peripapillary choroidal thickness. We observed that VM does not significantly change the subfoveal choroidal thickness, average peripapillary choroidal thickness, and the peripapillary choroidal thickness at eight other points (Figure 1). However, we believe that the temporary and slight changes in the peripapillary choroid during a VM may cause minimal vascular dysregulation and local vasospasm. These vascular changes and local vasospasm may also affect the optic nerve blood supply and morphology during regular or forced VM.
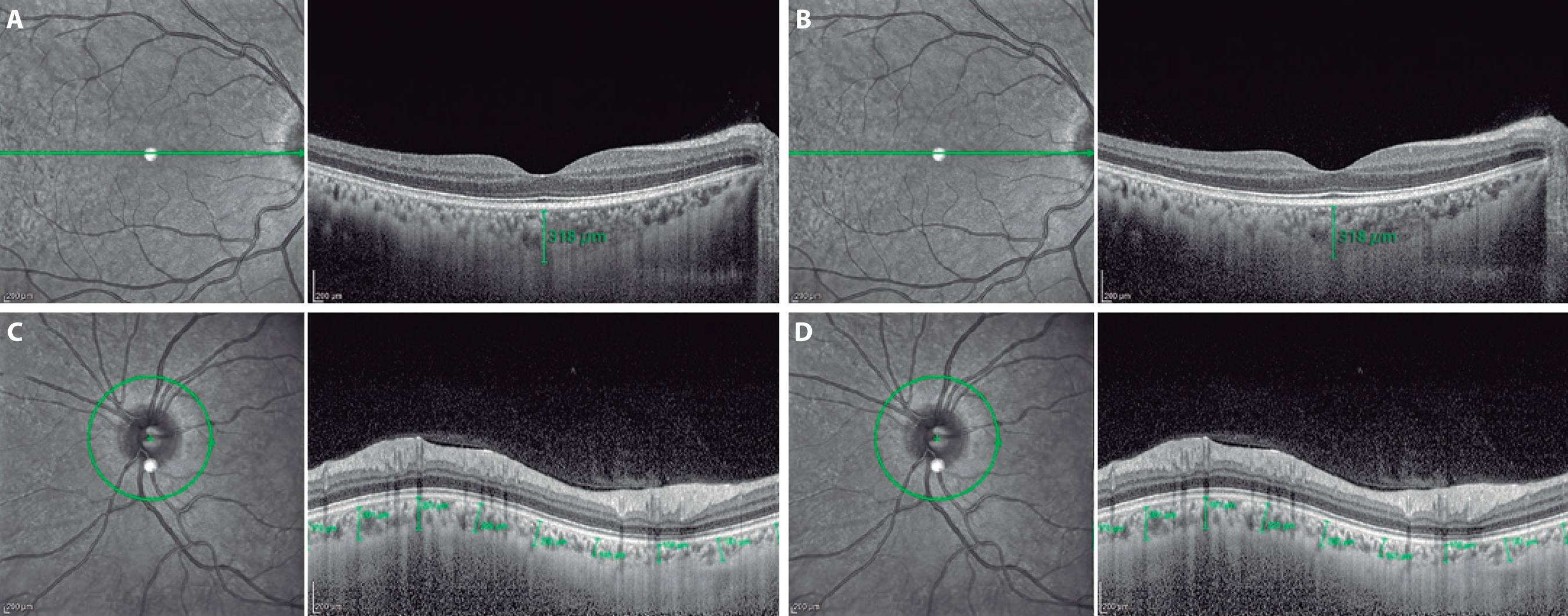
Figure 1 Subfoveal choroidal thickness measurements of a subject's right eye (A and B) and peripapillary choroidal thickness measurements of another subject's left eye (C and D) measured in a resting position and during a Valsalva maneuver as assessed using a Spectralis® spectral-domain optical coherence tomography with the enhanced depth imaging modality.
To our knowledge, there is only one report on the effect of VM on optic disc morphology in the existing literature. Zhang et al.(26) showed that the VM-associated short-term increase in cerebrospinal fluid pressure was significantly larger than the simultaneous short-term increase in IOP. This led to a VM-associated decrease or reversal of the trans-lamina cribrosa pressure difference, which corresponded to a decrease in the optic cup-related parameters and an increase in the neuroretinal rim-related parameters as observed via three-dimensional confocal laser scanning tomography of the optic nerve head. In our study, we observed that the cup volume decreases and the horizontal cup-to-disc ratio increases significantly during a VM. However, VM did not have any significant influence on the optic disc area, rim area, cup area, cup-to-disc area ratio, vertical cup-to-disc ratio, rim volume, or nerve head volume (Figure 2). We believe that the significant increase in the horizontal cup-to-disc ratio and the nonsignificant decrease in the vertical cup-to-disc ratio might be caused by the nonhomogenous structure of the optic nerve head. The superior and inferior neuroretinal rims are thicker than the nasal and temporal neuroretinal rims. During a VM, the vertical cup-to-disc ratio decreases, and this may cause a significant increase in the horizontal cup-to-disc ratio, leading to an ellipsoid shape in the cup area. Our findings showed that VM affects the optic nerve head morphology transiently, and these changes may prove important in further our understanding of the pathogenesis of GON.
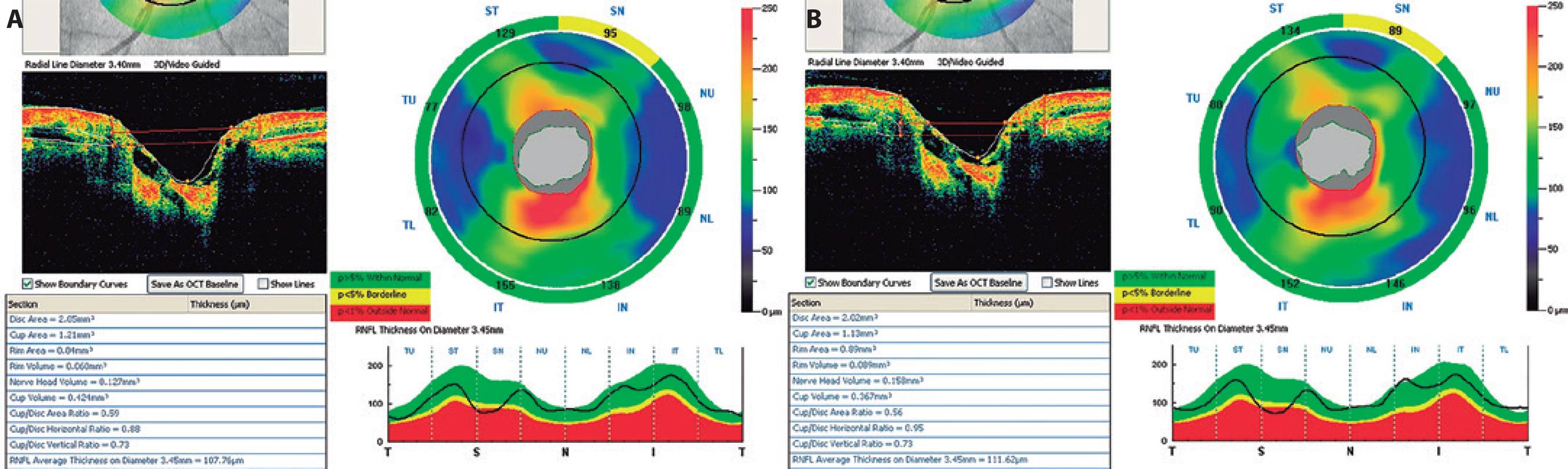
Figure 2 Optic disc parameters of a subject's right eye measured in a resting position (A) and during a Valsalva maneuver (B) using the RTVue® spectral-domain optical coherence tomography. The horizontal C/D ratio increased significantly.
In summary, VM may cause transient changes in IOP, optic disc morphology, and anterior chamber parameters. We believe that the increase in IOP is not related to changes in the posterior choroid. Anterior chamber changes, the increase in the anterior choroidal thickness, and the increase in the episcleral venous pressure are probably more related to this phenomenon. Changes in the optic nerve head, IOP, and anterior chamber parameters may affect the optic nerve blood supply, and these changes may cause local vasospasm or vascular dysregulation during regular or forced VM. Further long-term studies are required to detect a causal relationship between VM and GON.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

