INTRODUCTION
Major causes for developing a macular hole (MH) include anteroposterior forces applied by vitreofoveal traction and tangential traction from involutional changes in the inner retina(1-4). Kelly and Wendell(5) reported the first successful closure of MH with pars plana vitrectomy (PPV); since then, advances in vitreoretinal surgery have contributed to successful postoperative restoration of the foveal microstructure and restoration of visual function in most patients(6-10). Although anatomical closure of MH may be applicable to almost all patients undergoing surgical intervention, significant improvement in visual acuity can be achieved in approximately 70% of cases(8-13). Macular atrophy may be responsible for inferior visual outcomes; therefore, the foveal microstructure has to be well identified in patients with MH prior to any surgical intervention.
Spectral-domain optical coherence tomography (SD-OCT) is a non-contact, non-invasive, laser interferometry technique, which captures high-resolution cross-sectional images in vivo and is used in diagnosing numerous macular diseases. Using SD-OCT imaging in the diagnosis of MH augments clinical staging by enabling visualization of the foveal and vitreous microstructure and the tractional association between them and calculating macular hole dimensions. Manual quantification of MH width and height can be provided using SD-OCT, which mainly identifies the presence of perifoveal cystoid edema and vitreomacular traction (VMT) and the integrity of perifoveal external retinal microstructures. The impact of preoperative OCT measurements on diagnosing or staging idiopathic MH and its predictive value for postoperative anatomical and functional outcomes have been previously investigated(14-24). However, to the best of our knowledge, no study has elucidated the association between preoperative macular hole volume (MHV) and postoperative macular atrophy as a tool for predicting postoperative visual outcome. This study aimed to evaluate the association between MHV and postoperative central macular thickness (CMT) using SD-OCT.
METHODS
Thirty-three eyes of 30 patients with a diagnosis of large full-thickness idiopathic MH with or without VMT who underwent chromovitrectomy combined with internal limiting membrane (ILM) peeling and intraocular sulfur hexafluoride (SF6) tamponade in our Retina Unit between January 2010 and December 2012 were included in this study. Medical charts of the eyes were retrospectively reviewed, and all the results of complete ophthalmological examinations, including SD-OCT (Spectralis II HRA+OCT, Heidelberg Engineering Inc., Heidelberg, Germany) that were performed during pre- and postoperative visits, were recorded for each participant. Patients with systemic diseases that could affect visual acuity and patients who had a medical history of any ophthalmic surgery, except for cataract extraction, were excluded. Informed consent conforming to the Declaration of Helsinki was obtained from each study participant, and the study protocol was approved by the local ethics committee (approval number: 2013/43-16).
All eyes underwent 4-port 23-gauge PPV combined with ILM peeling and intraocular 20% SF6 tamponade. Detachment of the posterior hyaloid was induced by suction using a vitrectomy probe around the optic disc if necessary, and epiretinal membranes were removed if present. ILM peeling within a fovea-centered circular area of approximately two optic disc diameters was performed on visualizing ILM with brilliant blue dye. Twenty percent of SF6 was used as intraocular gas tamponade in all cases, and postoperative face-down positioning for 5-7 days was recommended. All subjects underwent complete ophthalmological examination, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopy, ocular tonometry, dilated fundoscopy, and CMT assessment using SD-OCT that were preoperatively and postoperatively performed.
MHV was measured on the basis of preoperative SD-OCT, which captured the widest cross-sectional image of the hole. Because the outer plexiform layer (OPL) and outer nuclear layer (ONL) junctions are located just beneath the foveal contour, OPL was first marked in all preoperative macular scans for each study participant. For the purpose of this study, we pointed out the OPL level along both sides of the MH, and a border line connecting these points was drawn to estimate the possible location of the foveal contour using SD-OCT that captured the widest cross-sectional image of the hole. The space remaining under the imaginary border line of OPL was described as MHV (Figure 1). The aspect of such spaces had the shape of a conical frustum; thus, we calculated MHV for each study participant according to the formula used for calculating the volume of a truncated cone (Figure 2).
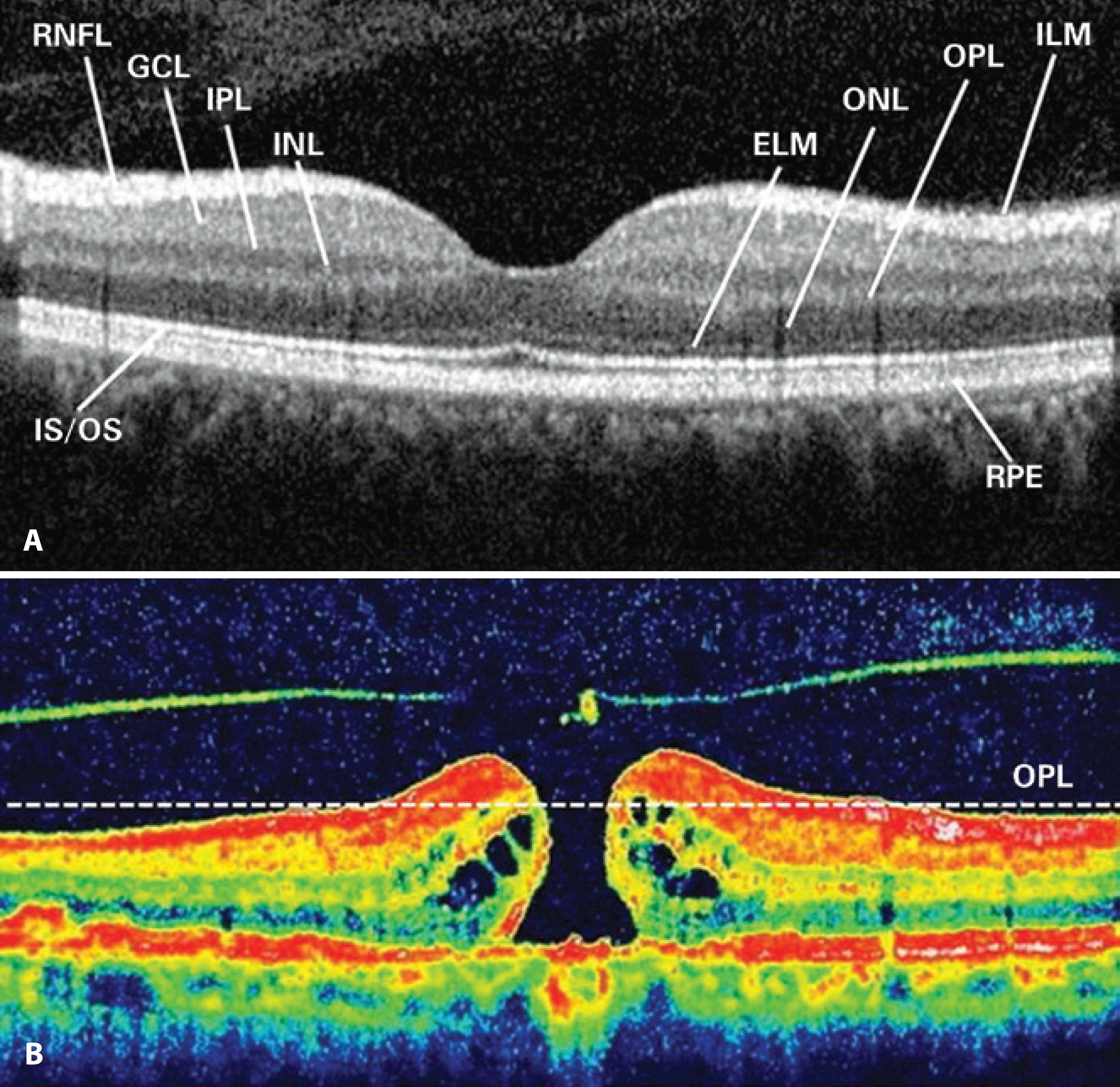
Figure 1 Retinal layers are shown in an SD-OCT image (A). An imaginary border line demonstrating the outer plexiform layer (OPL) was drawn to estimate the possible localization of the foveal contour on SD-OCT that captured the widest cross-sectional image of the hole (B).
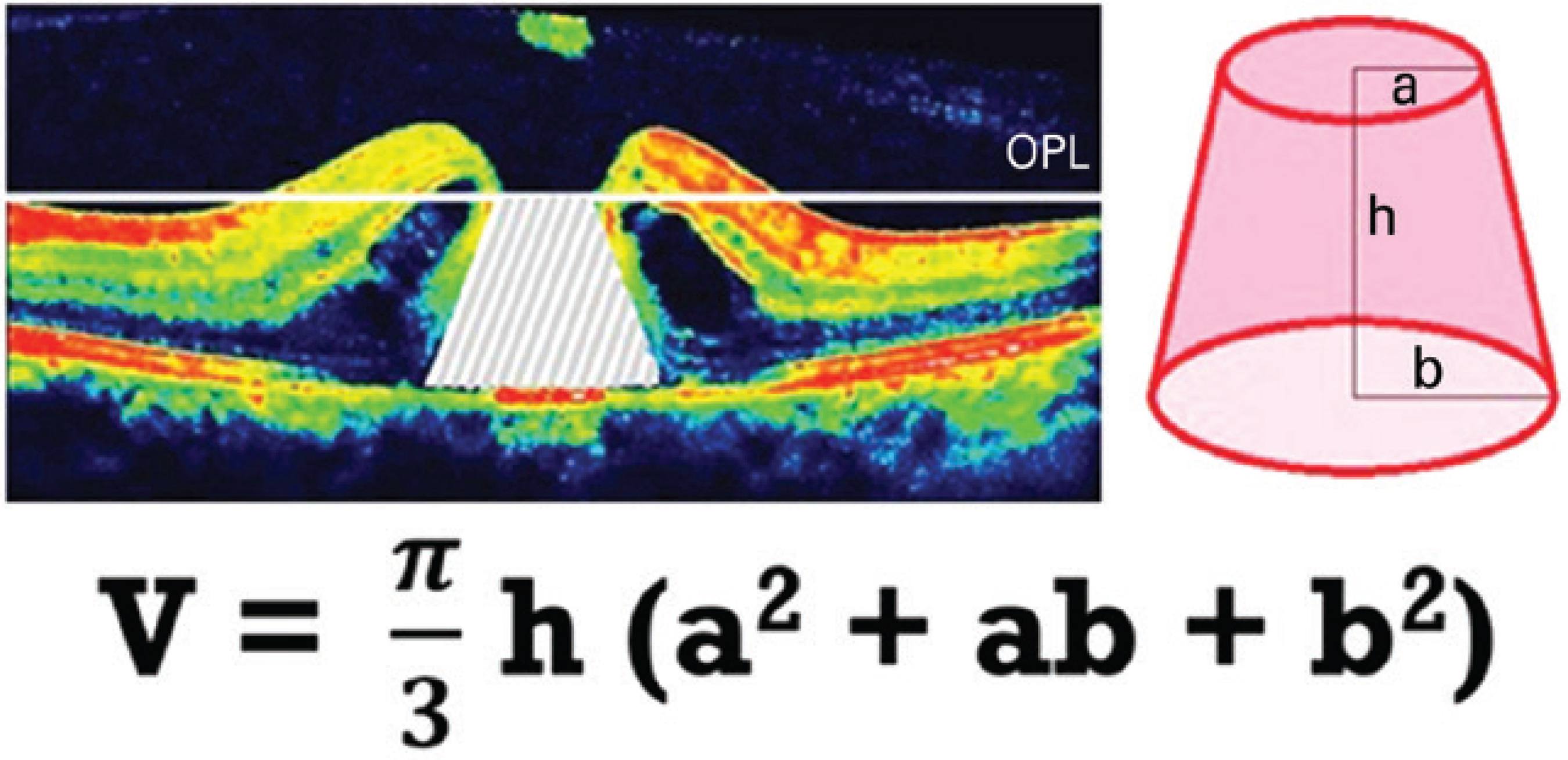
Figure 2 Calculation of the macular hole volume (MHV) according to the formula that gives the volume of a truncated cone.
The data were stored in a computerized database and analyzed using Statistical Package for Scientific Studies for Windows v.16.0 (SPSS Inc., Chicago, IL, USA). Normal distribution analysis of the data was performed using the Kolmogorov-Smirnov test; moreover, the chi-square test, the paired t-test, Student's t-test, and the Mann-Whitney U test were performed for statistical analyses. Pearson's correlation coefficient test was also used to analyze the association among MHV, postoperative CMT, and pre- and postoperative BCVA scores. A p value of <0.05 was considered statistically significant.
RESULTS
Of the 30 patients with a mean age of 63.9 ± 11.0 (range, 15-76) years, 18 (60.0%) were females and 12 (40.0%) were males. Three participants (10.0%) had diabetes mellitus and five (16.7%) had hypertension; however, none of the subjects demonstrated signs of retinopathy. Thirteen eyes (39.4%) were pseudophakic and 20 (60.6%) were phakic. Mean preoperative BCVA, intraocular pressure (IOP), and MHV were identified as 0.99 ± 0.36 (range, 0.3-2.0) logMAR, 14.7 ± 2.1 (range, 11-20) mmHg, and 0.139 ± 0.076 (range, 0.004-0.318) mm3, respectively. Surgery was performed within 6 months of the onset of clinical symptoms; thus, MH diagnosis was established soon after. Perifoveally located intraretinal cysts relevant to a newly developed MH were found on preoperative macular SD-OCT of all participants. These scans revealed VMT in 16 eyes (48.5%) and a true retinal operculum in 10 eyes (30.3%) preoperatively. Although early anatomical success was achieved with MH surgery in all cases, MH recurred in four eyes (12.1%) after the mean follow-up of 16.3 ± 14.3 (range, 3-50) months. Demographic and ophthalmological findings are summarized in tables 1 and 2.
Table 1 Demographic and initial ophthalmological findings
| All study eyes (n=33) | Eyes with c-MH (n=29) | Eyes with r-MH (n=4) | p value | |
|---|---|---|---|---|
| Age (years) | 63.900 ± 11.000 | 61.800 ± 14.400 | 70.200 ± 4.500 | 0.139 |
| Preoperative BCVA (logMAR) | 0.990 ± 0.360 | 0.930 ± 0.330 | 1.400 ± 0.350 | 0.013* |
| Preoperative IOP (mmHg) | 14.700 ± 2.100 | 14.900 ± 2.200 | 13.700 ± 1.000 | 0.322 |
| Macular hole volume (mm3) | 0.139 ± 0.076 | 0.138 ± 0.076 | 0.149 ± 0.075 | 0.777 |
| Gender | 0.643 | |||
| Female | 60.0% | 61.5% | 50.0% | |
| Male | 40.0% | 38.5% | 50.0% | |
| Concomitant disease | ||||
| Diabetes mellitus | 10.0% | 11.5% | - | 0.500 |
| Hypertension | 16.7% | 19.2% | - | 0.367 |
| Preoperative VMT | ||||
| VMT | 16 (48.5%) | 16 (55.2%) | - | 0.038* |
| A true retinal operculum | 10 (30.3%) | 10 (34.5%) | - | 0.159 |
| Follow-up (months) | 16.3 ± 14.3 | 16.3 ± 14.4 | 15.8 ± 15.2 | 0.939 |
c-MH= closed macular hole; r-MH= recurrent macular hole; BCVA= best-corrected visual acuity; IOP= intraocular pressure; VMT= vitromacular traction;
*= Statistical significance.
Table 2 Postoperative ophthalmologic findings in eyes with and without successful surgerym
| All study eyes (n=33) | Eyes with c-MH (n=29) | Eyes with r-MH (n=4) | p value | |
|---|---|---|---|---|
| Follow-up (months) | 16.30 ± 14.3 | 16.30 ± 14.40 | 15.80 ± 15.2 | 0.939 |
| Postoperative BCVA (logMAR) | 0.64 ± 0.38 | 0.51 ± 0.31 | 1.60 ± 0.0 | <0.001* |
| Postoperative IOP (mmHg) | 14.60 ± 1.90 | 14.70 ± 1.90 | 14.00 ± 1.8 | 0.502 |
| Postoperative CMT (µm) | 187.60 ± 67.80 | |||
| Reestablishment of ELM | 20 (60.6%) | 20 (69.0%) | - | 0.008* |
| Reestablishment of EZ | 13 (39.4%) | 13 (44.8%) | - | 0.085 |
| Reestablishment of COST line | 8 (24.2%) | 8 (27.6%) | - | 0.227 |
| Reestablishment of RPE band | 28 (84.8%) | 25 (86.2%) | 3 (75.0%) | 0.558 |
c-MH= closed macular hole; r-MH= recurrent macular hole; BCVA= best-corrected visual acuity; IOP= intraocular pressure; CMT= central macular thickness; ELM= external limiting membrane; EZ= ellipsoid zone; COST= cone outer segment tips; RPE= retinal pigment epithelium;
*= Statistical significanc
Mean BCVA and IOP were found to be 0.64 ± 0.38 (range, 0.0-1.6) logMAR and 14.6 ± 1.9 (range, 11-20) mmHg (p<0.001 and p=0.723), respectively, at the last follow-up visit. When four eyes with recurrent disease were excluded, mean BCVA and CMT were found to be 0.51 ± 0.31 (range, 0.0-1.6) logMAR and 187.6 ± 67.8 (range, 60-247) µm, respectively, at the last follow-up in the remaining 29 eyes. Mean preoperative BCVA and MHV were 1.40 ± 0.35 logMAR and 0.149 ± 0.075 mm3, respectively, in eyes with recurrent disease, whereas these scores were 0.93 ± 0.33 logMAR and 0.138 ± 0.076 mm3, respectively, in successfully treated eyes (p=0.013 and p=0.777, respectively).
VMT and a true retinal operculum were not found in any eyes with resistant MH; however, such vitreomacular interface abnormalities were present in 55.2% and 34.5% of eyes with closed MH (p=0.038 and p=0.159, respectively). Changes in BCVA, CMT, and IOP are shown in figure 3. While abnormalities were preoperatively present in the foveal ellipsoid zone (EZ) of all participants, SD-OCT revealed re-establishment of EZ in 13 eyes (39.4%). There was no correlation between preoperative MHV and postoperative EZ re-establishment (p=0.713). However, better postoperative BCVA scores were observed in patients with re-established EZ (p=0.002).
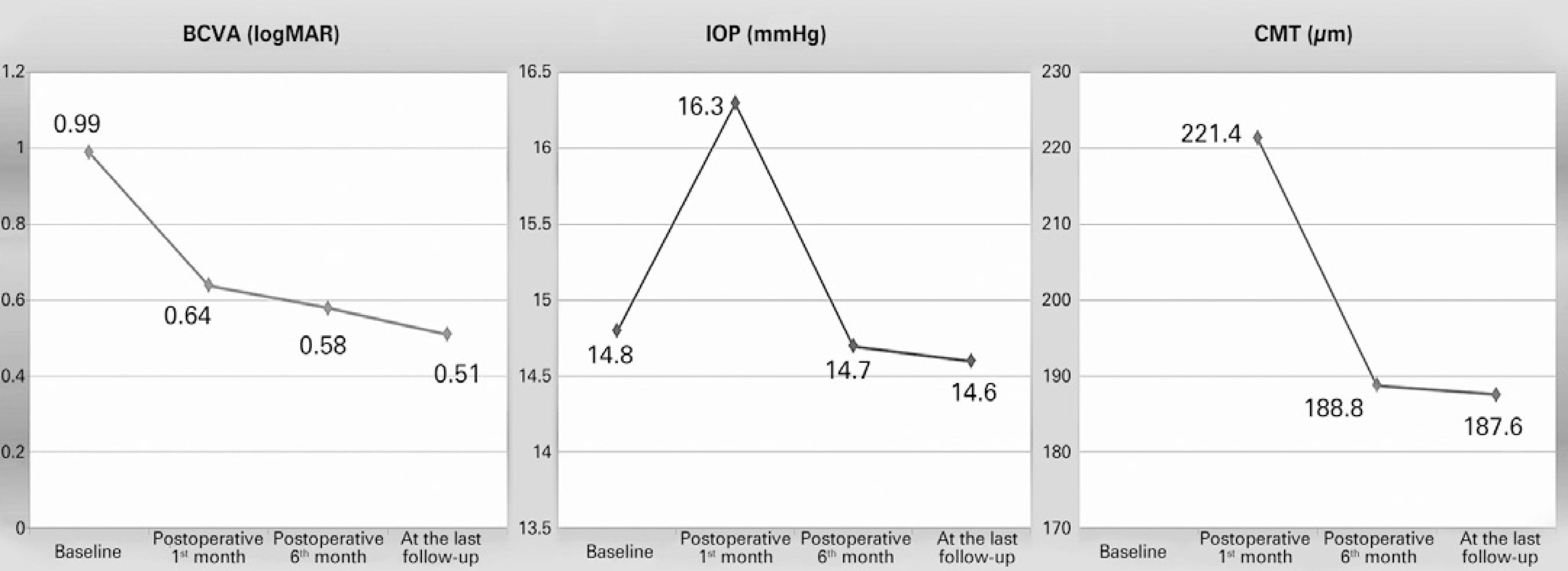
Figure 3 Changes in best-corrected visual acuity (BCVA), intraocular pressure (IOP), and central macular thickness (CMT).
Macular SD-OCT scans that were performed at the last follow-up visit also depicted the re-establishment of the external limiting membrane (ELM) in 20 eyes (60.6%), cone outer segment tip (COST) line in eight eyes (24.2%), and retinal pigment epithelium (RPE) band in 28 eyes (84.8%). Preoperative MHV was not found to be correlated with the re-establishment of ELM, COST line, or RPE band (p=0.268, p=0.283, and p=0.920, respectively). No statistical correlation was found between final BCVA and re-establishment of RPE band (p=0.411); however, reformed ELM and COST line provided a better visual prognosis in cases undergoing MH surgery (p=0.003 and p=0.005, respectively). Postoperative ocular features are summarized in table 2.
Although no statistical correlations were found between MHV and postoperative BCVA (p=0.588) and between MHV and disease recurrence (p=0.544), a negative correlation was present between MHV and CMT that was measured at the last follow-up (p=0.040). Furthermore, there was a strong correlation between pre- and postoperative BCVA scores (p<0.001). The results of Pearson's correlation coefficient test for the parameters studied are given in table 3.
Table 3 Pearson’s correlation coefficient test results for studied parameters
| Studied parameters | p value | r value | |
|---|---|---|---|
| Macular hole volume | Preoperative BCVA (logMAR) | 0.294 | 0.188 |
| Macular hole volume | Final BCVA (logMAR) | 0.588 | 0.113 |
| Preoperative BCVA (logMAR) | Postoperative BCVA (logMAR) | <0.001* | 0.660 |
| Macular hole volume | Postoperative CMT in cases with c-MH (n=29) | 0.040* | -0.383 |
BCVA= best-corrected visual acuity; CMT= central macular thickness; c-MH= closed macular hole;
*= statistical significance.
DISCUSSION
Recent advances in MH surgery techniques have contributed to more successful postoperative anatomical and associated functional prognoses(6-11). However, anatomical closure of a hole does not always correlate with a good functional result(6,17,18). Although various preoperative SD-OCT parameters have been examined to estimate postoperative visual prognosis, studies have reported the lack of any consistent volumetric or structural feature that is closely correlated with anatomical and functional surgical successes(15-18). In this study, there was no statistical difference in MHV measurements between eyes with and without disease recurrence; however, lower preoperative BCVA scores were found in eyes with relapsing MH. Furthermore, in our study population, preoperative SD-OCT revealed that neither VMT nor a true retinal operculum was found in any of the eyes with a recurrent hole.
Defects of foveal photoreceptors and abnormalities of external retinal microstructures that can be observed in SD-OCT images of surgically treated eyes with MH have been studied, with the goal of defining any association between such problems and the development of poorer vision. Furthermore, a significant correlation between the presence of such abnormalities and postoperative visual prognosis has been recently reported(16-20). In this study, MHV was not found to be associated with the postoperative re-establishment of any of the studied parameters of external retinal microstructure. Improved RPE band integrity on successful MH surgery was also not found to be associated with final BCVA scores; however, re-establishment of ELM, EZ, and COST line was associated with a better visual prognosis.
On the contrary, some published studies have reported a negative correlation between preoperative MH dimensions measured using OCT and postoperative visual recovery(21-26). The MH base diameter, which was measured at the RPE level, and the minimum diameter of the hole, which represented the shortest extent of MH, appear to constitute prognostic factors for postoperative anatomical and functional outcomes. Freeman et al.(6), similarly found that MH with smaller diameters were associated with a better visual prognosis. Moreover, a hole form factor (HFF) was calculated from the base and minimum diameters to estimate the postoperative anatomical success rate. Ullrich et al.(21) reported significantly larger base diameters and greater minimum MH diameters in cases with recurrent holes. Furthermore, postoperative visual prognosis was found to be negatively correlated with both the base and minimum diameters of the hole. The authors also stated that all cases with HFF of >0.9 were surgically treated with a single intervention; however, the anatomical success rate was 67% in patients with HFF of <0.5.
Ruiz-Moreno et al.(22) reported the sensitivity of various parameters used to estimate visual prognosis after MH surgery. They demonstrated that postoperative BCVA was statistically correlated with base and minimum diameters of MH, the MH index, which is defined as the ratio of the hole height to the base diameter, and the tractional hole index, which is defined as the ratio of the maximum height to the minimum diameter of MH. Base area, base diameter, top area, top diameter, minimum diameter, MH height, and MHV were found to be significantly correlated with initial BCVA in a computer algorithm-supported study by Xu et al(23). Moreover, they reported a significant correlation between BCVA at postoperative sixth month and MHV, base area and diameter, as well as height-to-base diameter ratio; however, after performing multivariate analyses, only base area and MHV were found to be significant predictors of six-month postoperative BCVA.
Pilli et al.(26) recommended performing ILM peeling to achieve a better visual outcome because a close correlation between inner macular volume and BCVA was observed. In our study, although no statistical correlations were found between MHV and postoperative BCVA or MHV and disease recurrence, a negative correlation was present between MHV and CMT measured at the last follow-up (p=0.04, r=-0.383). Because postoperative CMT scores were within the favorable normal range in the entire study population, except in cases with recurring MH, lower CMT scores were considered to be associated with a more severe macular atrophy (Figure 4). Visual acuity is directly related to both the extent and centricity of the macular atrophy(27,28). However, preoperative BCVA score was the only factor consistently affecting postoperative BCVA in our study population.
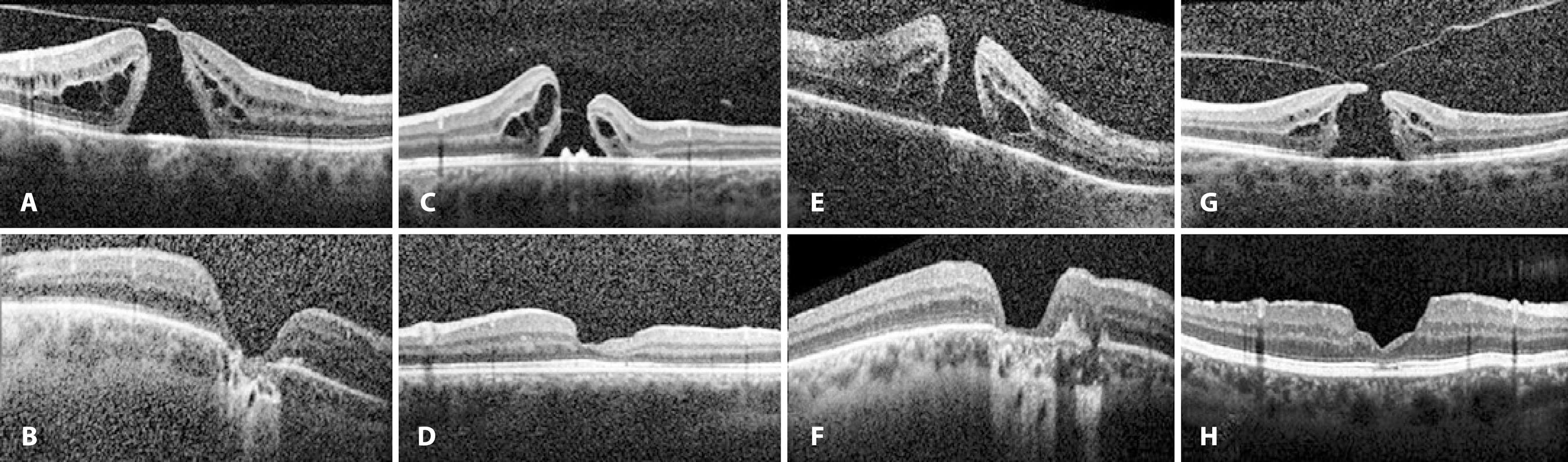
Figure 4 Preoperative (a, c, e, and g) and postoperative (b, d, f, and h) SD-OCT of patients with macular atrophy after 4-port 23-gauge PPV combined with ILM peeling and intraocular 20% SF6 tamponade.
The major limitation of this study was the lack of the use of real-time three-dimensional volumetric analysis of MH. Although the shape of each MH varies in its microarchitecture, we used the widest two-dimensional cross-sectional SD-OCT image of all studied holes. Nevertheless, we believe that within a close range of deviation, every MHV, regardless of the varying shape of the hole, may simply be predicted using the parameters of the formula that gives the volume of a truncated cone. The second limitation was that 20% of our study participants had concomitant diabetes mellitus, hypertension, or both. However, we deliberately selected the surgically treated cases with a newly developed idiopathic MH who did not have any other retinal abnormalities, including retinopathies and a previous history of retinal detachment. In addition, the coexistence of diabetes and/or hypertension may result in some cellular and microarchitectural changes within the retinal tissue, even in cases with no signs of retinopathy. Finally, the retrospective nature of this study and the lack of a large study population reflect other limitations.
Visual acuity may not improve as expected in some patients even when anatomical closure of the hole is attained following MH surgery. This may be related with postoperative macular atrophy, which can be diagnosed on the basis of a reduced CMT score on SD-OCT. In this study, greater MHV was associated with lower postoperative CMT scores. To the best of our knowledge, this is the first study investigating the association between preoperative MHV and macular atrophy. Long-term prospective trials with large cohorts are required to assess the correlation of macular volumetric features with postoperative anatomical and functional prognoses.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

