INTRODUCTION
Glaucoma is characterized by a progressive loss of retinal ganglion cells (RGCs) and atrophy of the optic nerve, leading to a characteristic pattern of visual field loss(1). It is the leading cause of irreversible visual loss worldwide(2), and without treatment, glaucoma can cause blindness(3). Primary open-angle glaucoma (POAG) is the most common type of glaucoma(4). While the pathogenesis of POAG is not yet known, there are several known risk factors, one of which is elevated intraocular pressure (IOP)(5). To decrease the IOP with this treatment slows down the progression of the disease(1). However, visual loss may still continue despite reducing the IOP in some patients(1).
Another type of glaucoma is normal-tension glaucoma (NTG), in which there is optic nerve degeneration without IOP elevation. Patients with statistically normal IOP who develop the characteristic changes of progressive retinal nerve fiber layer (RNFL) loss, RGC loss, and visual field defects are grouped as having NTG, an important subset of open-angle glaucoma (OAG)(6). POAG and NTG patients form the subgroups of OAG patients.
Dementia, which affects 24 million people worldwide, with double the number of affected people every 20 years, is an important health problem for the aging population(7). It is a term used to describe a group of conditions that can affect a person's ability to think, remember, understand, make judgments, communicate, and interact socially(8). Alzheimer-type dementia (ATD) is known as the most widespread form of dementia and is an important health problem in every country(9). It is a progressive neurodegenerative disorder characterized by cognitive deterioration and deterioration in memory, changes in personality, behavioral disturbances, and impaired ability to perform the activities of daily life(10).
Glaucoma and dementia (especially ATD) share several features. Both become more severe with advanced age, and they occur more frequently in women than in men(11,12). Common genetic risk factors have been reported in ATD and glaucoma, and similar pathological changes in the optic nerves of glaucoma patients and the brains of patients with ATD have been demonstrated(13). At the molecular level, caspase activation was shown in a rat study of chronic ocular hypertension to induce abnormal amyloid precursor protein formation, which is the key event in the pathogenesis of ATD(14).
Both ATD and glaucoma demonstrate early structural changes in the visual cortex and lateral geniculate nucleus. Both diseases affect magnocellular visual processing(15). Spectral domain-optical coherence tomography (SD-OCT) can demonstrate these early changes, so it is reasonable to use OCT when assessing for cognitive decline in glaucoma patients to analyze the possible correlations between OCT measures and cognitive parameters.
Although several clinical studies have demonstrated an increased prevalence of glaucoma in dementia patients(16), large population-based studies have not revealed an association between glaucoma and dementia(17). Since there have been very few studies of the relationship between glaucoma and dementia, more studies are necessary to firmly establish this relationship. Since the MMSE is one of the most frequently used screening tools for the assessment of cognitive function, the aim of our study was to determine whether there are differences among the POAG, NTG, and control (C) groups in terms of MMSE scores, and also to assess the relationship between the MMSE scores and SD-OCT parameters.
METHODS
The study was performed in compliance with the Helsinki Declaration and with the approval of the Ethics Committee of the Antalya Education and Research Hospital. A total of 60 people, including 20 POAG patients (aged between 44 and 73 years), 20 NTG patients (aged between 45 and 73 years), and 20 healthy C participants (aged between 47 and 69 years), participated in this study, and the age and gender proportions of the groups were similar. We recruited our patients in the hospital outpatient setting. C subjects meeting the inclusion criteria were recruited in the same setting from the neurology and ophthalmology outpatient clinics. Glaucoma patients were taking at least one topical medication (beta-blockers, carbonic anhydrase inhibitors, prostaglandin analogs, sympathomimetic drugs, and parasympathomimetic drugs). All of our participants had graduated from elementary school. Since most of the subjects in the patient and C groups had attained this level of education, illiterate subjects and high school and university graduates were excluded to provide homogeneity. The majority of the subjects were on a similar level of socioeconomic status. Patients who had neurological diseases that could have affected cognitive performance, such as ATD, vascular dementia, and mild cognitive impairment (MCI), were excluded from this study. None of the patients had subjective complaints concerning cognitive impairment. Further, at the time of the study, none of the participants had been using systemic medications that could have affected cognition such as benzodiazepines, opiates, tricyclic antidepressants, anticonvulsants, and dopamine agonists, etc. Subjects who did not meet these criteria were excluded. Subjects with moderate-severe depression and chronic systemic diseases such as diabetes mellitus, arthritis, hypertension, heart disease, stroke, and cancer were excluded. Additionally, patients who smoked cigarettes, had a best-corrected visual acuity of less than 1.0 according to Snellen chart, or had eye diseases other than glaucoma that could have affected RNFL and ganglion cell-inner plexiform layer (GC-IPL) thicknesses, such as macular degeneration and optic neuropathy, were excluded from this study.
Ophthalmological examination
All participants in this study underwent an ophthalmological examination including visual acuity assessment with a Snellen chart, IOP with Goldman's applanation tonometer after application of a local anesthetic (hydrochloric proxymetacaine 0.5%), measurement of central corneal thickness (CCT) with an optic pachymeter (Lenstar LS 900; Haag-Streit, Koeniz, Switzerland), slit-lamp-assisted biomicroscopy of the anterior and posterior segments of the eye, gonioscopy, and photography of the fundus (Visucam NM-FA; Carl Zeiss Meditec Inc., Oberkochen, Germany). SD-OCT (Cirrus HD OCT model 5000; Carl Zeiss Meditec Inc., Dublin, CA, USA) was used to measure the GC-IPL (macular cube 512 × 128) and RNFL (optic disc cube 200 × 200) thicknesses. The examination was concluded with a check of the visual fields using a static perimeter apparatus type Octopus 900 (Haag-Streit). These examinations were used for the glaucoma diagnosis and classification. The diagnostic criteria for POAG were high IOP (>21 mmHg, corrected by corneal thickness), normal iridocorneal open angle, glaucomatous changes in the visual field with optic nerve cupping, and the absence of other optic neuropathies. Except for evidence of high IOP (≤21 mmHg), we used the same diagnostic criteria of POAG to diagnose NTG. All POAG and NTG diagnoses were applied according to the guidelines of the European Glaucoma Society(18).
Neurological examination
Detailed neurological examination, including the MMSE test which evaluates cognitive function, was applied to all participants. All of the patients had intact neurological examination findings, including those of the motor, sensory, and cerebellar systems and the cranial nerves, except for the optic nerve. The MMSE is the most commonly used test for evaluating cognitive function and scanning for dementia. Orientation, attention, memory, language, and shape copying are evaluated in this test. The maximum number of points is 30. The study for the validity and reliability of the MMSE in Turkey was performed by Güngen(19) and his associates in 2002, and the cut-off value was determined to be 23/24. This test is affected by education, and is considered to be reliable for identifying the degree of mild dementia. There is also a modified version for uneducated people. The MMSE is used for both diagnosis and treatment follow-up. Long-term memory, short-term memory, attention span, calculation, the naming of items, performance of a task with three steps, reading, writing, and assembling abilities are all measured in this test.
Statistical analysis
All statistical analyses were performed using IBM SPSS 20 for Windows (IBM Corp., Armonk, NY, USA). IOP, CCT, mean deviation (MD), RNFL thickness, GC-IPL thickness, and MMSE scores were compared among the groups. The normality and variance homogeneity assumptions of the continuous variables for the parametric methods were evaluated by the Shapiro-Wilks and Levene's tests, respectively. The IOP and CCT were provided normality and homogeneity of variance assumptions. For this reason, the one-way analysis of variance (ANOVA) test was used to compare the differences among the three groups for each variable. Non-parametric tests, including the Kruskal-Wallis H test and the Mann-Whitney U test, were used for the other variables, which were not provided assumptions for the parametric tests (RNFL thickness, GC-IPL thickness, MD, and MMSE scores). Correlations among the variables were assessed using Spearman's correlation coefficients. P <0.05 was regarded as statistically significant.
RESULTS
The mean ages of group one (POAG), group two (NTG), and group three (C) were 59.8 ± 10.1, 61.9 ± 9.8, and 60.1 ± 8.5 years, respectively. There were no significant differences with regard to age among the groups (p =0.348). All subjects involved in the study were Caucasian. There were 11 males and nine females in the POAG group, 11 males and nine females in the NTG group, and 10 males and 10 females in the C group (p =0.935) (Table 1). The mean IOP value was 16.2 ± 2.9 mmHg in the POAG group, 14.7 ± 1.9 mmHg in the NTG group, and 14.8 ± 2.9 mmHg in the C group (p =0.270).
The CCT was 545.6 ± 29.7 µm in the POAG group, 519 ± 25.7 µm in the NTG group, and 551.9 ± 26.2 µm in the C group. The differences observed between the NTG group and the two other groups were statistically significant (p <0.001). When we compared the groups pairwise, we found statistically significant differences between the NTG and POAG groups (p =0.001), and the NTG and healthy C groups (p <0.001). The MD in the worse eye was 4.8 ± 3.1 dB in the POAG group, 7.9 ± 3.8 dB in the NTG group, and 1.2 ± 1.1 dB in the C group; there were significant differences among the three groups (p <0.001). The MD in the better eye was 3.5 ± 2.1 dB in the POAG group, 4.6 ± 3.1 dB in the NTG group, and 0.7 ± 0.7 dB in the C group; there were significant differences among the three groups (p =0.001). The IOP, CCT, MD in the worse-eye, and MD in the better-eye measurement values, are shown in table 1.
Table 1 Demographic and clinical characteristics of patients and control subjects
| POAG | NTG | Control | ||
|---|---|---|---|---|
| n=20 | n=20 | n=20 | p value | |
| Age+ (years) | 59.8 ± 10.1 | 61.9 ± 9.8 | 60.1 ± 8.5 | 0.348 |
| Sex (M/F)* | 11/9 | 11/9 | 10/10 | 0.935 |
| TO+ | 15.5 ± 1.9 | 16.2 ± 2.9 | 14.8 ± 2.9 | 0.270 |
| CCT+ | 545.6 ± 29.7 | 519.0 ± 25.7 | 551.9 ± 26.2 | <0.001 |
| MD worse eye** | 4.8 ± 3.1 | 7.9 ± 3.8 | 1.2 ± 1.1 | <0.001 |
| MD better eye** | 3.5 ± 2.1 | 4.6 ± 3.1 | 0.7 ± 0.7 | 0.001 |
POAG= primary open-angle glaucoma; NTG= normotensive glaucoma; M= male; F= female; TO= tension ocular; CCT= central corneal thickness; MD= mean defect;
+= one-way ANOVA test with Bonferroni correction;
*= Chi-square test;
**= Kruskal-Wallis test.
Among the POAG patients, three were taking beta-blockers, seven were taking prostaglandin analogs, four were taking beta-blockers plus carbonic anhydrase inhibitors, two were taking beta-blockers plus prostaglandin analogs, two were taking beta-blockers plus sympathomimetic drugs, one was taking beta-blockers plus carbonic anhydrase inhibitors as well as parasympathomimetic drugs, and one was taking beta-blockers plus carbonic anhydrase inhibitors plus sympathomimetic drugs. Among the NTG patients, three were taking beta-blockers, eight were taking prostaglandin analogs, two were taking sympathomimetic drugs, two were taking beta-blockers plus carbonic anhydrase inhibitors, four were taking beta-blockers and prostaglandin analogs, and one was taking beta-blockers plus sympathomimetic drugs.
The mean RNFL thickness measurements were 85.2 ± 14.7 µm for the POAG group, 76.8 ± 10.3µm for the NTG group, and 91.4 ± 7.7 µm for the C group; statistically significant differences were found among the three groups (p <0.001). When the groups were compared pairwise, statistically significant differences were found between the NTG and POAG groups (p =0.034), and the NTG and C groups (p <0.001). The difference between the POAG and C groups was not statistically significant (p =0.102). These measurements are shown in table 2.
Table 2 Comparison of the mean RNFL, GC-IPL, and MMSE complex among the POAG, NTG, and control groups
| POAG | NTG | Control | ||
|---|---|---|---|---|
| n=20 | n=20 | n=20 | p value | |
| RNFL (mean)* | 85.2 ± 14.7 | 76.8 ± 10.3 | 91.4 ± 7.7 | <0.001 |
| GC-IPL (mean)* | 77.5 ± 9.7 | 73.4 ± 7.8 | 78.8 ± 3.8 | 0.085 |
| MMSE* | 26.1 ± 1.4 | 25.7 ± 2.3 | 28.8 ± 0.9 | <0.001 |
RNFL= retinal nerve fiber layer; GC-IPL= ganglion cell-inner plexiform layer; MMSE= mini-mental state examination; POAG= primary open-angle glaucoma; NTG= normotensive glaucoma;
*= Kruskal-Wallis test.
The mean GC-IPL measurement was 77.5 ± 9.7 µm in the POAG group, 73.4 ± 7.8 µm in the NTG group, and 78.8 ± 3.8 µm in the control group. No statistically significant difference could be found when all the groups were compared with each other (p =0.085). However, when the groups were compared pairwise, the difference between the NTG and C groups was statistically significant (p =0.023). When we compared the POAG group with the NTG and C groups separately, no significant differences were found (p =0.143 and p =0.714, respectively). The above measurements are shown in table 2.
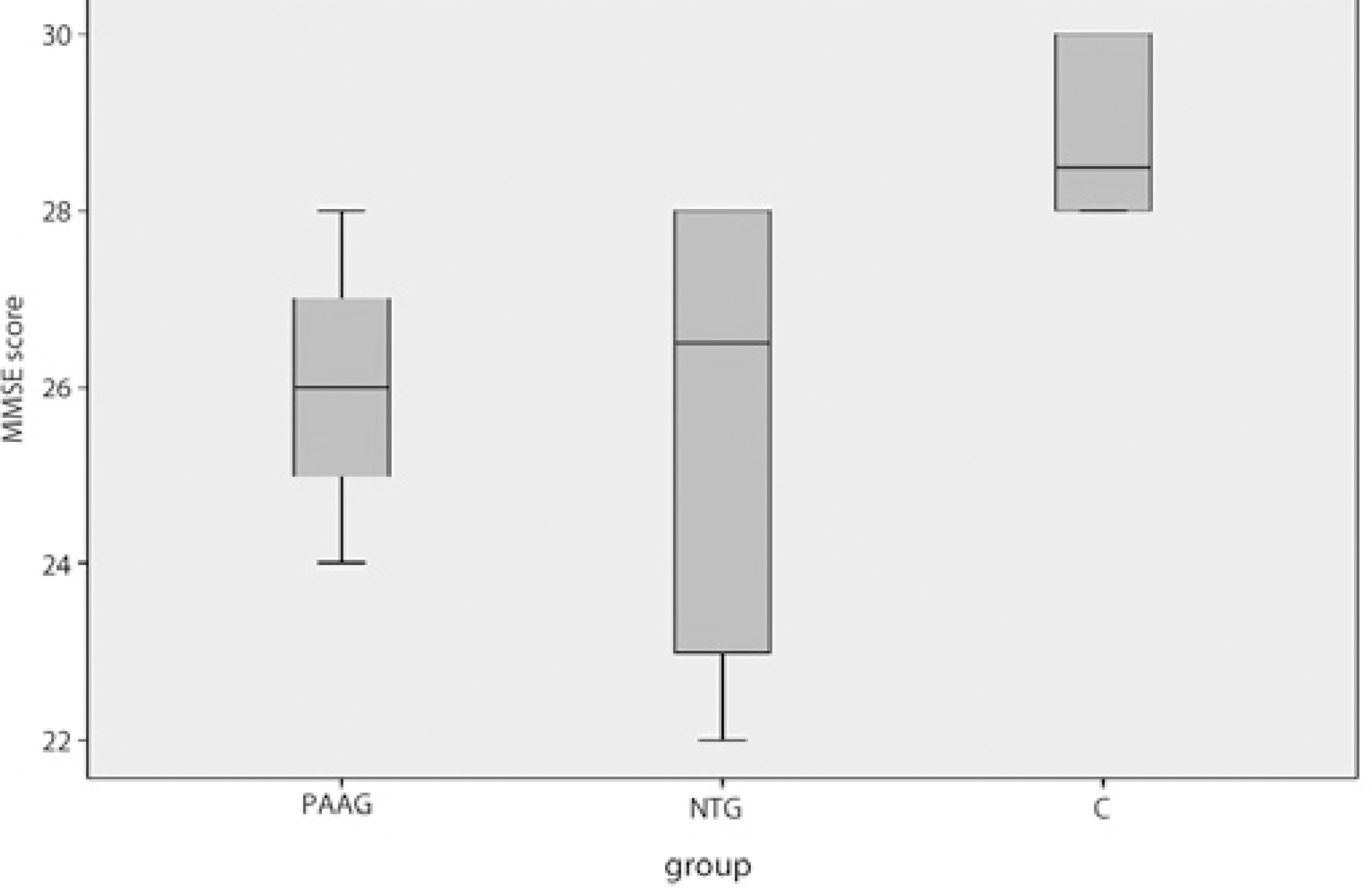
The MMSE scores were 26.1 ± 1.4 in the POAG group, 25.7 ± 2.3 in the NTG group, and 28.8 ± 0.9 in the C group. When all three scores were compared together, the differences between the measurements were statistically significant (p <0.001). The measurements are shown in table 2. When the scores were compared pairwise, a statistically significant difference was found between both the NTG and C groups, and the POAG and C groups; however, the difference was not statistically significant between the NTG and POAG groups. The measurements are shown in table 3. A significant correlation was not found between the MMSE scores and either the RNFL (p =0.385) or GC-IPL (p =0.813) thicknesses.
Table 3 Pairwise comparison of the MMSE scores between the control subjects and the POAG and NTG patients
| Control (n=20) MMSE: 28.8 ± 0.9 |
POAG (n=20) MMSE: 26.1 ± 1.4 |
p=0.001* |
| NTG (n=20) MMSE: 25.7 ± 2.3 |
p<0.001* |
MMSE= mini-mental state examination; POAG= primary open-angle glaucoma; NTG= normotesive glaucoma;
*= Mann-Whitney U test.
DISCUSSION
In the present study, it was necessary to determine if there were any differences in the MMSE scores (same age and gender) of the three groups (20 POAG, 20 NTG, and 20 healthy C participants). In this study, the hypothesis was that lower cognitive scores in glaucoma patients were anticipated. The pathogenesis of the dementia group diseases, such as ATD and MCI, which cause lower MMSE scores, is similar to that of glaucoma. Since both diseases have noticeable neuron loss, it evident that there is a relationship of some kind.
Studies have shown a higher incidence of glaucoma among ATD patients. In a previous study, the rate of glaucoma was found to be 25.9% in ATD patients and 5.2% in controls(17). Tamura et al. found that 23.8% of patients with ATD had glaucoma, while only 9.9% of the age-matched controls had glaucoma(1). From these data, it was proposed that the optic nerve of patients with ATD may be less resistant to elevated IOP(15). Based on this proposition, we suspected that in the early stages of ATD, lower IOP may cause glaucomatous optic neuropathy; therefore, the NTG patients could be expected to attain lower MMSE scores. However, we did not find a significant difference in the MMSE scores between the POAG and NTG groups.
Few studies have explored cognitive impairment in glaucoma patients. Yochim et al. found cognitive impairment in 44% of 41 older glaucoma patients(20). Similarly, we found lower MMSE scores in the glaucoma patients than in the healthy C group. Hagerman et al. found that 32% of patients with low vision had cognitive impairment as determined by the MMSE(21). In contrast, Cumurcu et al. showed no significant differences in the MMSE score among pseudoexfoliative glaucoma patients, POAG patients, and healthy controls(22).
The dementia group of disorders (ATD, vascular dementia, mixed dementia) causes cognitive impairment(23). The MMSE is used to evaluate the cognitive condition of a person and scanning of dementia. Personal orientation, attention to detail, language ability, and the copying of shapes are evaluated in this test, and 30 is the maximum possible score. Further, general cognitive function can be assessed with the MoCA (Montreal Cognitive Assessment), which provides a more comprehensive assessment than MMSE. Executive function, short-term memory, attention, language skills, and visuospatial processing are the categories included in the MoCA test(24). Since the educational level of our patients was low, we could not perform the MoCA test in the present study. In our study, we found statistically significant different MMSE scores among the three groups (26.1 ± 1.4 in the POAG group, 25.7 ± 2.3 in the NTG group, and 28.8 ± 0.9 in the C group; p <0.001). Similarly, Jefferis et al. found lower MMSE scores in glaucoma patients as opposed to healthy controls(25). Since MMSE requires intact vision for eight of the 30 points, they also studied the MMSE scores of their subjects by extracting the part of the MMSE that requires intact vision. At this time, they found no significant difference in the MMSE scores between the glaucoma patients and healthy controls. In our study, we excluded patients with vision less than 1.0 according to Snellen chart, to minimize the effect of vision on MMSE scores.
Although we excluded patients with vision less than 1.0 according to the Snellen chart, vision may still have affected the MMSE scores in glaucoma patients, since glaucoma affects the magnocellular visual pathway. Visual function mediated by this part of the brain is difficult to assess by conventional ophthalmological examination. Motion process and contrast sensitivity are parts of these visual processes(26). In addition, ATD patients may have structural defects in the magnocellular visual pathway even in the absence of plaques and neurofibrillary tangles in these brain areas(27).
In our study, we found that the mean thicknesses of RNFL and GC-IPL in both the glaucoma groups were thinner, as expected, compared with the normal C group. These results are consistent with those of previous studies(27,28). The CCT values were found to be significantly thinner in the NTG group than in the other groups (p <0.05). These results are also consistent with those from the available literature(29). We did not find any significant correlations between the MMSE score and either the RNFL or GC-IPL thicknesses. The literature did not reveal any studies with similar glaucoma and dementia parameters for glaucoma patients. In some studies performed on dementia-group diseases causing cognitive impairment, a significant correlation was found between the MMSE score and RNFL thickness(30,31). In addition, in a study by Bayhan et al., a significant correlation was(32) found between the MMSE score and the mean GC-IPL thickness. Conversely, in another study, no correlation was found between the MMSE score and RNFL thickness(33).
To minimize the effects of vision on the MMSE score, because we did not include patients with a visual acuity of less than 1.0, most of our patients had early-stage glaucoma. Since POAG patients are at an earlier stage of glaucoma than NTG patients, even though the GC-IPL and RNFL thicknesses were lower in these two groups than in the C group, this difference was not statistically significant. We found that NTG patients had lower MMSE scores than POAG patients. We believe that although there was no statistically significant difference of the MMSE scores between the NTG and POAG patients, the lower values of MMSE in NTG patients might be attributed to the later stage of glaucoma in those patients. Figure 1 shows a box plot graph of the MMSE scores in each group.
Some research has shown that glaucoma has some of the same characteristics as ATD, which is the most common cause of dementia(1). They are both chronic neurodegenerative diseases that are closely related to aging, and both progress very slowly. Recently, Yoneda et al.(34) suggested that beta-amyloid and tau (neurofibrillary tangles), which have significance in ATD pathology, may also have important roles in glaucoma pathology. They also found a significant decrease in the levels of beta-amyloid and a significant increase in the level of tau in the vitreous of glaucoma patients when compared with that in the vitreous of a healthy control group. In a previous study, when ATD patients were compared with a control group, a significant decrease was found in the beta-amyloid levels of the cerebrospinal fluid, as well as a significant increase in the level of tau(35). Based on these findings, the neurodegenerative process causing neuron loss in glaucoma may have a mechanism similar to the process causing the same pathology in ATD. In addition, it was found that beta-amyloid accumulates in the RGCs of rats with glaucoma induced for experimental purposes(14). In another experimental glaucoma study performed on rats, a hypothesis was proposed that chronic beta-amyloid neurotoxicity at the molecular level causes the death of retinal neurons and is similar to the death of neurons in the brains of ATD patients(36).
There were some limitations in this study. The number of subjects (60) was relatively low, and the glaucoma drugs used by the patients were not taken into account. Although all the patients in this study had good visual acuity, we could not confirm the effect of visual function on the MMSE scores. The absence of complete neuropsychologic data is another limitation. Unfortunately, we were unable to perform more sensitive tests to evaluate the cognitive status of the patients, such as the MoCA, due to the low educational level of the participants. This could be considered a limitation of our study. Undoubtedly, longitudinal studies should be performed to obtain more data on this topic. A strength of this study is that the neurologist who performed the neurologic examinations on the patients was completely unaware of the ophthalmological diagnoses of the patients.
In conclusion, since the glaucoma group of diseases and the dementia group of diseases share similar neurodegenerative processes affecting cognitive impairment, our findings support our hypothesis. Since glaucoma patients have lower cognitive performance, glaucoma can take place in the group of neurodegenerative diseases. Therefore, it is incumbent upon the ophthalmologist to refer glaucoma patients to a neurologist.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

