INTRODUCTION
Migraine is a chronic disease involving both neurological and vascular system abnormalities and is characterized by single-sided, episodic attacks of headache. Gastrointestinal complaints and sensitivity to bright light and loud noises may accompany this headache(1), and migraine occurs most frequently between the ages of 35 and 45 years(2). Although the etiology of migraine is unknown, there are some theories for its pathogenesis. At the end of the 1930s, Graham and Wolff identified a dilatation in the temporal artery during migraine attacks as a cause for the headache. Wolff advocated that the aura preceding migraine symptoms is due to vasospasms that reduce the blood flow to the brain(3).
Several studies have shown that vascular changes occur in the ocular system during migraine attacks. Cerebral blood flow in the occipital hemisphere has been shown to diminish during migraine attacks, especially in migraine with aura. Hypoperfusion has been reported to affect the retina and the optic nerve, as well as result in ganglion cell loss(4). The retinal nerve fiber layer (RNFL) contains the axons of the retinal ganglion cells. Therefore, measurement of the mean peripapillary RNFL thickness is expected to provide benefits for monitoring the progressive loss of ganglion cells and axons in migraine patients.
Optical coherence tomography (OCT) can be used to obtain high-resolution images of the anterior and posterior segments of the eye. Today, modern technological OCT devices are employed for the in vivo quantitative measurement of the peripapillary RNFL, GCL, and choroid layer in various neuro-ophthalmological diseases. The reduction in RNFL thickness reflects the loss of ganglion cells and axons in migraine patients(4-6).
We expected the retina and optic nerve to be affected in recurrent migraine attacks with aura due to hypoperfusion. We evaluated the thicknesses of the peripapillary RNFL, ganglion cell complex, subfoveal choroid, and choroid layer at six distinct points using spectral domain (SD)-OCT.
METHODS
This cross-sectional study included patients who were diagnosed with migraine with aura at Fatih University Faculty of Medicine (study group), together with age-matched normal individuals (control group). Informed consent, which was obtained from all individuals, was prepared in accordance with the principles of the Declaration of Helsinki and was approved by the local ethics committee.
The study group was selected from patients who were diagnosed with migraine with aura (45 patients) according to the 2004 criteria of the International Headache Society (IHS)(2). A detailed anamnesis, including the history of headache, was obtained from each patient. Physical and neurological examinations were performed, and radiological examinations (computed tomography or magnetic resonance imaging) were performed as required. The control group in our study consisted of 45 age- and sex-matched healthy volunteers.
The inclusion criteria were as follows: age between 18 and 45 years, spherical or cylindrical refractory error less than +/-2 D, visual acuity of 20/20, intraocular pressure (IOP) <18 mmHg, cup-to-disc ratio <0.4, and no ophthalmological pathologies. The exclusion criteria were as follows: glaucoma, cataracts, previous eye surgery, systemic diseases such as diabetes, hypertension, history of prophylactic migraine treatment, including calcium channel blocker, beta blockers, and anti-epileptics, to avoid their pharmacological effects on the retina.
Complete ophthalmological examination, including visual acuity, anterior segment, anterior chamber angle, and fundus examinations, as well as IOP measurement, central corneal thickness (CCT) measurement, and OCT measurements, were performed for each patient at our Ophthalmology Department. Best-corrected visual acuity was recorded using Snellen charts. Ocular axial lengths and CCT were measured using an ultrasonic biometry and pachymetry device (US-4000 Echoscan; NIDEK, Gamagori, Japan). Peripapillary RNFL, central macular thickness (CMT), GCL, and choroidal thickness (CT) were measured using an SD-OCT device (RS-3000; NIDEK). SD-OCT measurements were performed between 09:00-12:00 am by the same person 30 min after the dilation of the pupils with 0.5% tropicamide solution. Only the scans that had a signal strength of at least 6 or above with good reliability were included in the analysis.
The peripapillary RNFL thickness was measured by circular scanning around the optic nerve in an area with a diameter of 3.4 mm. We recorded the RNFL thicknesses at the superior, inferior, temporal, and nasal quadrants, and the mean RNFL thickness values. CMT was measured at the superior and inferior GCL regions. For CT analysis, measurements were performed vertically between the outer hyperreflective border of the retinal pigment epithelial layer and the choroid-sclera border, at the subfoveal area, and at six extrafoveal areas (500 µm, 1000 µm, 1500 µm nasal side of the fovea, and 500 µm, 1000 µm, and 1500 µm temporal to the fovea). Data obtained from the migraine patients were compared with those from the control group.
Statistical analysis
Statistical analyses were performed on NCSS (Number Cruncher Statistical System) 2007 & PASS (Power Analysis and Sample Size) 2008 Statistical Software (Kaysville, UT, USA). Along with descriptive statistics (mean, standard deviation, median, frequency, ratio, minimum, and maximum), Student's t -test was used for the comparison of normally distributed variables between the two groups. A repeated measures test (repeated measures analysis of variance) was used for the comparison of normally distributed parameters within a group, and Bonferroni correction was used for the evaluation of dual comparisons. The comparison of qualitative data was performed with Yates' continuity correction test (Yates' chi-square test with correction). Significance was evaluated at the p <0.01 and p <0.05 levels.
RESULTS
The present study included 90 individuals; 73 (81.1%) of them were female and 17 (18.9%) were male. The mean age of the individuals was 35.9 ± 7.6 (19-45) years. There were 37 (82.8%) females and 8 (17.8%) males in the migraine group, and 36 (80%) females and 9 (20%) males in the control group. The mean age in the migraine group was 36.1 ± 6.5 (20-45) years and in the control group was 35.7 ± 8.6 (19-45) years. There was no significant difference between the groups with regard to age and sex (p =0.159, p =0.124, respectively).
For the migraine patients, the mean duration after diagnosis was 8.8 ± 6.9 (1-28) years, and the mean IOP in this group was 16 ± 3 (10-22) mmHg. The mean CCT measurement was 553.4 ± 31.8 (490-645) µm. The demographic and clinical characteristics of the migraine and control groups are given in table 1.
Table 1 Demographic and clinical characteristics of the migraine group
| N=45 | Mean ± SD (min-max) |
|---|---|
| Age (years) | 36.1 ± 6.5 (20-45) |
| Years since diagnosis (years) | 8.8 ± 6.9 ( 1-28) |
| IOP (mmHg) | 16.0 ± 3.0 (10-22) |
| CCT (μm) | 553.4 ± 31.8 (490-645) |
SD= standard deviation; IOP (mmHg): intraocular pressure; CCT= central corneal thickness
The mean CMT was 226.3 ± 12.4 µm in the migraine group and 226.6 ± 15.6 µm in the control group. The measurements of the axial length and CMT were not significantly different between the groups (p =0.907, p =0.929, respectively; Table 2). As for the measurements of the RNFL thickness, the temporal and nasal quadrant thicknesses were not statistically significantly different between the groups (p =0.298, p =0.395, respectively), whereas the mean quadrant thickness values were significantly lower in the migraine group than in the control group (p= 0.016). Similarly, the superior and inferior thickness measurements were significantly lower in the migraine group than in the control group (p =0.001, p =0.001, respectively; Table 2, Figure 1).
Table 2 Mean OCT analysis results of the migraine and control groups
| Groups | |||||
|---|---|---|---|---|---|
| Migraine group (n=45) |
Control group (n=45) |
||||
| Mean ± SD | Mean ± SD | *p value | |||
| AL (mm) | 23.2 ± 0.9 | 23.2 ± 0.8 | 0.907 | ||
| CMT (μm) | 226.3 ± 12.4 | 226.6 ± 15.6 | 0.929 | ||
| RNLF (μm) | |||||
| Mean | 104.1 ± 8.4 | 108.7 ± 9.4 | 0.016** | ||
| Superior | 107.0 ± 11.9 | 131.8 ± 18.3 | 0.001*** | ||
| Inferior | 101.6 ± 10.4 | 138.4 ±17.1 | 0.001*** | ||
| Temporal | 72.9 ± 10.3 | 75.3 ±11.5 | 0.298 | ||
| Nasal | 87.9 ± 15.1 | 85.3 ±14.0 | 0.395 | ||
| GCL (μm) | |||||
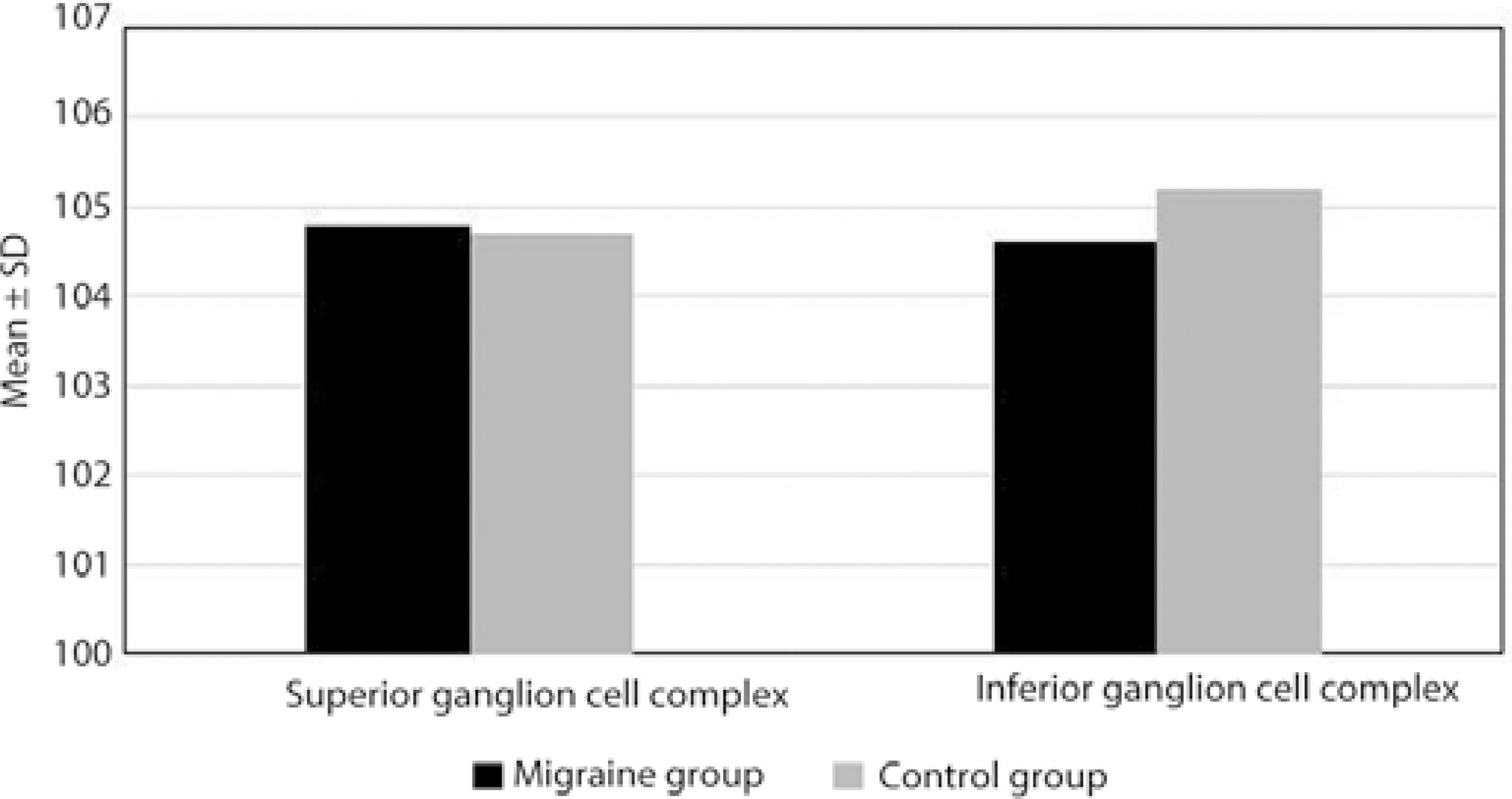
| Superior GCL | 104.8 ± 5.2 | 104.7 ±6.2 | 0.927 | ||
| Inferior GCL | 104.6 ±5.3 | 105.2 ±5.9 | 0.233 | ||
| CT (μm) | |||||
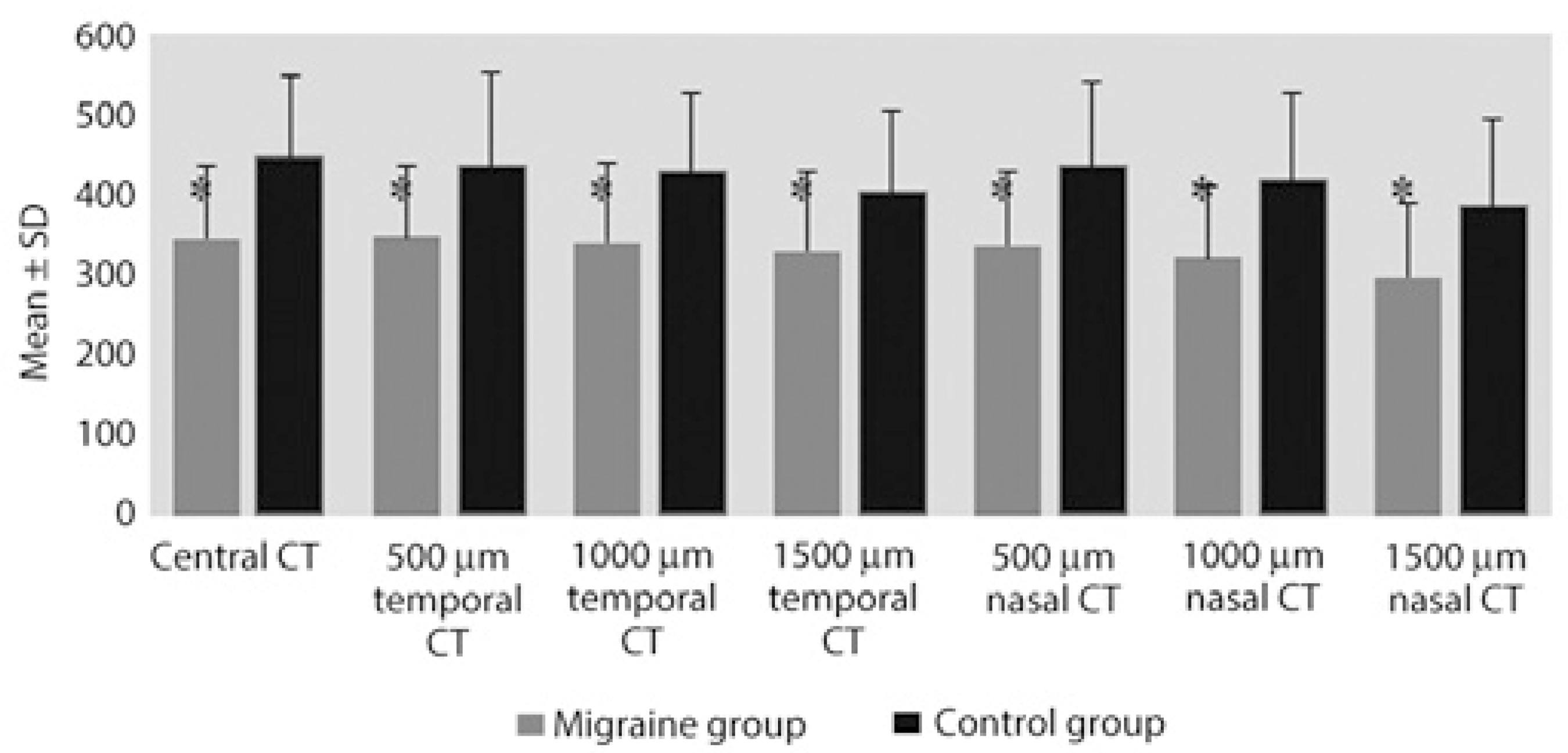
| SFCT | 343.0 ± 90.7 | 445.3 ± 101.6 | 0.001*** | ||
| Temporal 500 μm CT | 344.0 ± 90.5 | 433.0 ± 116.6 | 0.001*** | ||
| Temporal 1000 μm CT | 339.4 ± 97.1 | 425.3 ±98.4 | 0.001*** | ||
| Temporal 1500 μm CT | 328.5 ± 97.9 | 403.4 ±98.9 | 0.001*** | ||
| Nasal 500 μm CT | 335.7 ± 89.9 | 435.7 ± 104.2 | 0.001*** | ||
| Nasal 1000 μm CT | 320.1 ± 90.3 | 417.8 ± 104.9 | 0.001*** | ||
| Nasal 1500 μm CT | 296.8 ± 88.6 | 385.1 ± 107.6 | 0.001*** | ||
*= Student’s t-test;
**= p<0.05;
***= p<0.01.
SD= standard deviation; AL= axial length; CMT= central macular thickness; RNFL= retinal nerve fiber layer; GCL= ganglion cell layer thickness; CT= choroidal thickness; SFCT= subfoveal choroidal thickness.
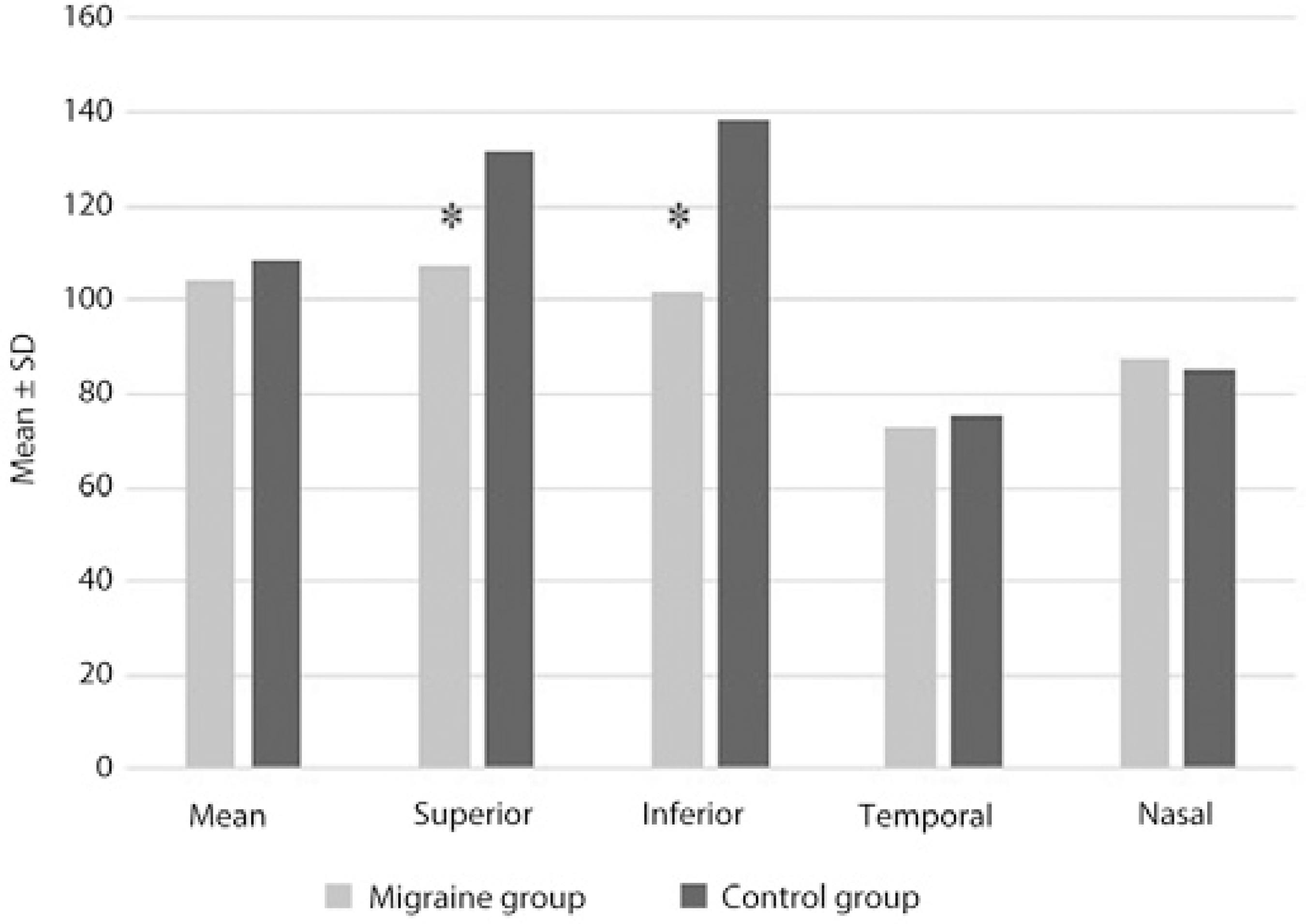
Figure 1 Measurements of the retinal nerve fiber layer thickness in the migraine and control groups.
The superior and inferior GCL thickness measurements were not statistically significantly different between the two groups (p =0.907, p =0.233, respectively; Table 2; Figure 2).
As for the CT measurements, the subfoveal, temporal, and nasal CT at 500 µm, 1000 µm, and 1500 µm were significantly thinner in the migraine group than in the control group (p =0.001; Table 2, Figures 3 and 4).
DISCUSSION
Migraine is a common neurological disease characterized by headaches together with transient focal symptoms called aura observed in some patients. Migraine patients with aura reportedly have increased rates of ischemic stroke, cardiac disease, intracerebral hemorrhage, and mortality(4,7). Endothelial and vascular smooth muscle dysfunction as well as hypercoagulability have been reported as possible responsible factors for the pathogenesis of migraine(8). Due to the transient cerebral vasospasm that occurs in migraine, fluctuations in perfusion during recurrent migraine attacks can lead to chronic retinal damage in the optic nerve head and the retina and eventually to ganglion cell death(4,9). Recurrent migraine attacks have been shown to induce chronic cerebral damage in several studies(10). Further, retinal infarcts due to the occlusion of the retinal artery have also been reported in migraine patients(11).
It has recently become possible to evaluate the thickness of the peripapillary RNFL and choroid layer using advanced OCT devices. This method has enabled the diagnosis and monitoring of various diseases. Today, RNFL analysis using OCT is commonly performed in patients with ocular hypertension and glaucoma(12,13). In our study, we compared the thicknesses of the RNFL, CMT, GCL, and choroid layer in migraine patients with aura to that in healthy individuals using SD-OCT.
In one study by Kara et al. in which Doppler ultrasound was used to demonstrate retinal vascular changes and perfusion in migraine patients, the resistance in the central retinal artery and posterior ciliary artery was found to be higher in migraine patients than in the control group(14).
In another study by Martinez et al., who compared 70 migraine patients and 53 normal individuals using OCT, the mean RNFL thickness was determined to be normal in migraine patients, but the RNFL thickness in the temporal quadrant was significantly thinner(4). Tan et al. evaluated RNFL thicknesses using laser polarimetry in their study, including 39 migraine patients, 15 with aura and 24 without aura, and 25 healthy individuals; they determined no reduction in the RNFL thickness, and they asserted that migraine had no effect on RNFL(15). Additionally, Sorkhabi et al. found that RNFL was thinner in the nasal quadrant in migraine patients(16).
In our study, while there was no statistically significant difference in the temporal and nasal quadrant RNFL thickness measurements between the groups (p >0.05), the mean RNFL thickness in migraine patients was found to be significantly lower compared with that in the control group (p <0.05). Similarly, the superior and inferior quadrant RNFL thicknesses were significantly lower in the migraine group than in the control group (p <0.05).
Various visual complications have been reported in migraine, such as visual field defects similar to that observed in glaucoma as well as retinal vasculopathy(17). Ischemic damage causes thinning in RNFL in glaucoma, and RNFL thickness measurements are used to evaluate the glaucomatous changes. Studies have shown that the peripapillary RNFL is consistently thinner in patients with glaucoma. Kook et al. observed thinning in RNFL in patients with normal-tension glaucoma (NTG) in the superior and inferior quadrants, consistent with our observation(18). Drance et al. determined that the progression of visual field loss was faster in NTG patients who also had migraines compared with that in NTG patients who did not have migraines(19)). Moreover, the reduction of RNFL thickness has also been reported in multiple sclerosis (MS), Alzheimer's disease, and Parkinson's disease(20-22). The GCL thickness could provide a better structural indicator of axonal loss compared with RNFL in certain optic neuropathies such as MS, non-arteritic anterior ischemic optic neuropathy, and compressive optic neuropathy. GCL analysis may provide a method for diagnosing and monitoring optic nerve disease(23).
In their study comparing female migraine patients to healthy women, Gipponi et al. did not find any difference in the foveal thickness and macular volume; however, they determined that superior RNFL and GCL thicknesses were significantly reduced only in migraine patients with aura but not in those without aura(24). In our study, we did not observe a significant difference in the CMT and GCL measurements of migraine patients with aura and healthy individuals (p >0.05).
The choroid layer is the most important vascular layer of the eye. Choroidal vascular insufficiency and reduction in CT result in the dysfunction of the retinal pigment epithelium and photoreceptor layers(25,26). In migraine, which is regarded as a neurovascular disease, the choroid layer can become thinner due to reduced blood flow in the central retinal and posterior ciliary arteries(27). Diseases that affect the retina lead to reduced thickness of the choroid layer.
In their study evaluating CT in diabetic retinopathy, Regatieri et al. determined that CT can vary depending on the severity of retinopathy caused by hypoxia in the retinal tissue, and they found that CT significantly decreased with increasing severity of retinopathy caused by hypoxia, especially in the presence of diabetic macular edema(28). Bourke et al. reported a correlation between untreated systemic hypertension and choroidopathy(29). The reduction of CT has been reported in a study with smokers, which was thought to be due to increased resistance in the retinal vasculature(30). Similarly, we observed that the subfoveal, temporal, and nasal CT measurements at 500 µm, 1000 µm, and 1500 µm were significantly lower in migraine patients compared with those in controls (p <0.01).
We found that the thicknesses of the RNFL and choroid layer were reduced in migraine patients compared with those in controls. Considering that patients with other diseases that could cause any retinal abnormalities, such as glaucoma (especially NTG) and systemic diseases, were not included in the study, this reduction is likely to be related to the migraine itself.
The limitations of our study are the exclusion of migraine patients without aura and the small number of cases in the study.
In conclusion, the RNFL and choroid layer thicknesses were determined to be thinner in migraine patients with aura compared with those in age-matched healthy subjects, and this is thought to be related to a progressive loss of ganglion cells and axons.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

