INTRODUCTION
Congenital cataracts are the major cause of preventable and/or treatable childhood blindness(1,2), accounting for 8%-39% of cases of childhood blindness(3). The worldwide prevalence of congenital cataracts has been estimated to be between 1 and 15 per 10,000 children(1).
Surgical treatment for congenital cataract has developed over the last few decades, allowing cataract removal in children younger than 1 year. Congenital cataract deprives the retina of light during periods of greater neural plasticity, and surgery is the primary treatment to prevent amblyopia; however, it only partially solves the problem because the aphakic eye has a refractive error that must be corrected(4,5).
Biometric calculation of a growing eye is challenging, and intraocular lenses (IOLs) implanted for emmetropia will produce a myopic shift with axial growth(6). Portable devices for biometric calculation may not be available for some of congenital/developmental cataract surgeons. Therefore, the selection of the IOL power at the time of implantation is based on tables of biometric measurements by age in normal eyes, which have biometric characteristics that may differ from the characteristics of eyes with cataracts, or on tables of biometric data in cataract eyes collected from populations in specific ethnicity (5,7).
The objective of this study was to evaluate the ocular axial length (AL) and keratometry (K) in Brazilian children with congenital/developmental cataract to assess the differences and evolution according to age as well as to establish functional models of AL and K in function of age and between K and AL.
METHODS
This retrospective observational study was approved by the Botucatu Medical School Ethics Committee; we analyzed the medical records of patients undergoing cataract surgery for congenital/developmental cataract at the Clinical Hospital of Botucatu Medical School, São Paulo, Brazil.
Prematurely born children as well as those with glaucoma, retinal disease, microphthalmia, traumatic cataract, pathological myopia, lens subluxation, or other ocular diseases were not included.
Ocular biometric data were obtained under general anesthesia by an experienced ophthalmologist, and cataract surgery was performed immediately after these measurements were made. The keratometric measurements were obtained using a regularly calibrated handheld autorefract keratometer (Retinomax K-Plus 2; Righton, Tokyo, Japan). AL measurement was performed with a contact ultrasonic biometer (model 1000A; Sonomed, New York, NY, USA). The ultrasound velocity used was 1532 m/s for the anterior chamber, 1641 m/s for the lens, and 1532 m/s for the vitreous.
All eyes with unilateral cataract and the left eyes in children with bilateral cataracts were selected and included in the analysis. In children with unilateral cataract, the affected eyes were compared with the healthy eye. The biometric data were transferred to Microsoft Excel 2007 (Redmond, WA, USA). The statistical analyses for the paired samples were performed using Student's t-test.
The assessment of the relationship between the child's age in months and the ocular biometric measurements was calculated with Pearson's correlations. The linear regression analysis using the logarithm of a patient's age as an independent variable was adjusted to obtain the estimates of the mean ocular parameters (K and AL). We considered P values <0.05 as statistically significant.
RESULTS
A total of 44 children were included in the study, and 28 (63%) were male. The median and mean age at the time of cataract surgery were 12.5 months and 27.3 months, respectively (range: 1.5-92 months). Twenty-nine children (66%) had bilateral cataracts, and 15 children (34%) had a unilateral cataract.
The eyes with unilateral cataract were not significantly different in terms of the mean AL and K with the left eyes in the bilateral cases (P>0.05) (Table 1).
Table 1 Axial length (AL) and mean keratometry (K) values according to the laterality of the cataracts
| Groups | n | Age (months) | AL (mm) | K (D) | |
|---|---|---|---|---|---|
| Unilateral | 15 | Mean ± SD | 27.7 ± 27.1 | 20.6 ± 2.0 | 44.8 ± 2.7 |
| Median | 18 | 20.8 | 44.5 | ||
| Range | 2-75 | 17.9-24.7 | 41.7-53.0 | ||
| Bilateral | 29 | Mean ± SD | 27.1 ± 27.7 | 20.6 ± 2.2 | 45.0 ± 2.4 |
| Median | 10 | 19.9 | 45.0 | ||
| Range | 1-92 | 17.3-25.0 | 40.0-51.0 | ||
| P value | 0.960 | 0.990 | 0.629 |
AL= axial length; K= keratometry; D= diopter; SD= standard deviation; Student's t-test.
The 44 analyzed eyes had a mean AL of 20.63 mm (range 17.27 mm- 24.96 mm) and a mean K of 44.94 D (range 40.00 D-53.00 D). The calculations of the mean, standard deviation, confidence interval, median, and variable range for AL and K according to the age groups are shown in table 2. The mean of AL increased with age, whereas the mean of K decreased. These relationships were statistically significant (P<0.001). If the AL observations were divided into those from children under and over 12 months old, the relative variability expressed by the coefficient of variation showed homogeneity of the response between the two age groups.
Table 2 Mean, standard deviation, confidence interval, median, and range of the axial length (AL) and keratometry (K) values according to the age group of 44 eyes with unilateral cataract and one randomly chosen eye from the bilateral cataract cases
| Age groups (months) | n | Mean ± SD | 95% CI | Median | Range |
|---|---|---|---|---|---|
| AL (mm) | |||||
| 0-5 | 9 | 18.45 ± 0.77 | 17.95-18.95 | 18.43 | 17.27-19.83 |
| 6-17 | 15 | 19.34 ± 0.90 | 18.89-19.79 | 19.64 | 17.85-21.22 |
| 18-59 | 11 | 22.19 ± 0.92 | 21.62-22.76 | 21.93 | 20.80-23.36 |
| 60-96 | 9 | 22.07 ± 1.53 | 22.08-23.06 | 22.74 | 20.82-24.96 |
| Total | 44 | 20.63 ± 2.11 | 20.01-21.25 | 19.97 | 17.27-24.96 |
| K (D) | |||||
| 0-5 | 9 | 46.86 ± 3.30 | 44.71-49.01 | 46.00 | 43.00-53.00 |
| 6-17 | 15 | 44.83 ± 1.86 | 43.89-45.77 | 45.00 | 41.75-49.00 |
| 18-59 | 11 | 44.18 ± 1.40 | 43.28-45.08 | 44.25 | 41.75-46.25 |
| 60-96 | 9 | 44.16 ± 2.60 | 42.47-45.85 | 44.50 | 40.00-49.00 |
| Total | 44 | 44.94 ± 2.44 | 44.22-45.66 | 44.75 | 40.00-53.00 |
AL= axial length; K= keratometry; D= diopter; SD= standard deviation; CI= confidence interval; Student's t-test.
The linear regression analyses performed using the natural logarithm (ln) of the patient's age (in months) as an independent variable are shown below by the following equations: AL=16.66 + (1.476 × ln age);R2=0.74, P <0.001 and K=46.97 + (-0.752 × ln age); R2=0.14,P< 0.001.
A graphical representation of AL and K according to the patients' age and the fitted curves derived from the model above are shown in figures 1 and 2. AL and K change markedly with an increase in age. The highest rates of ocular axial growth and corneal flattening are observed in the first 6 months of life.
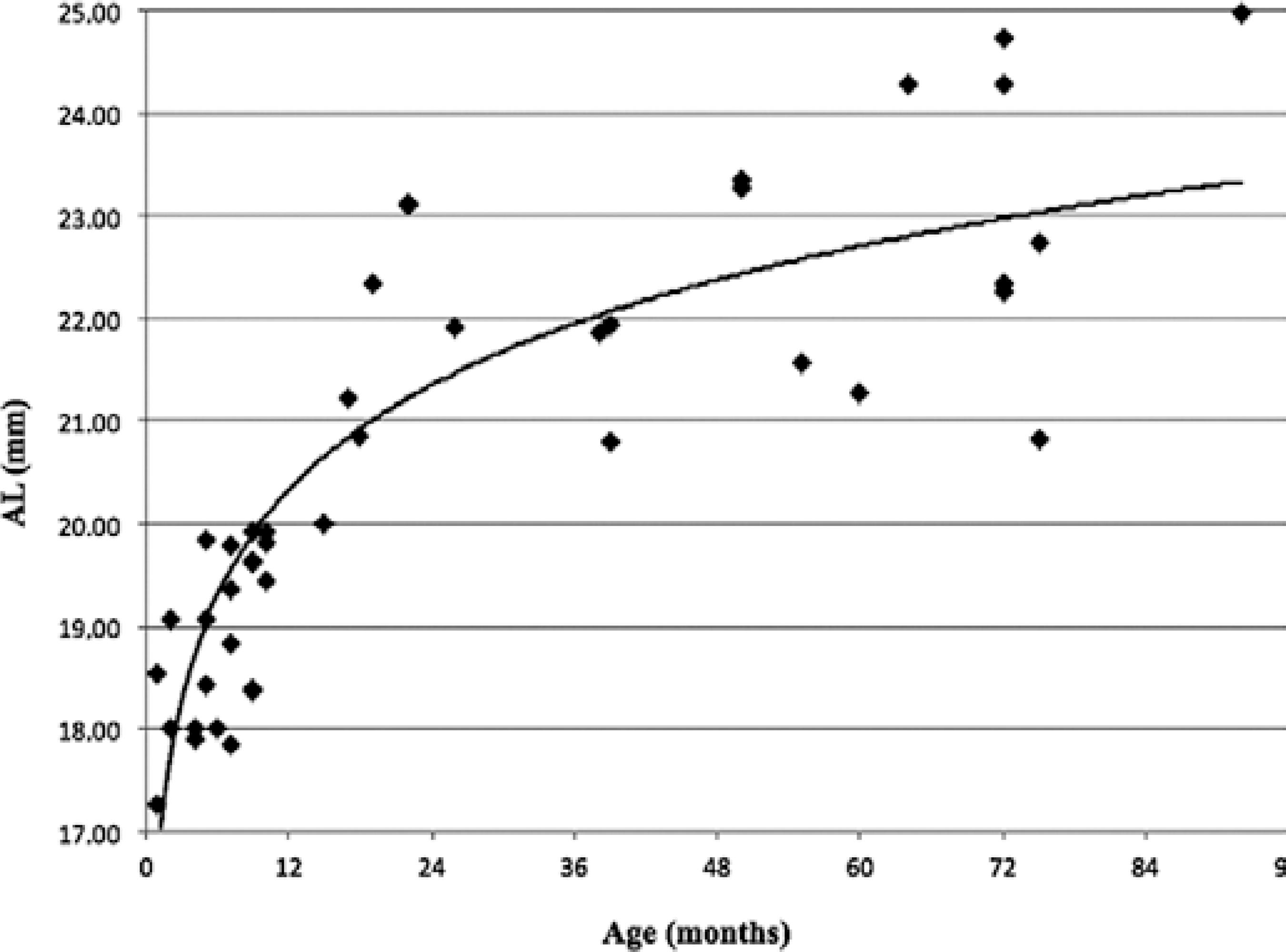
Figure 1 Distribution of axial length (AL) values of 44 eyes of children with unilateral cataracts and randomly selected eyes in the bilateral cases.
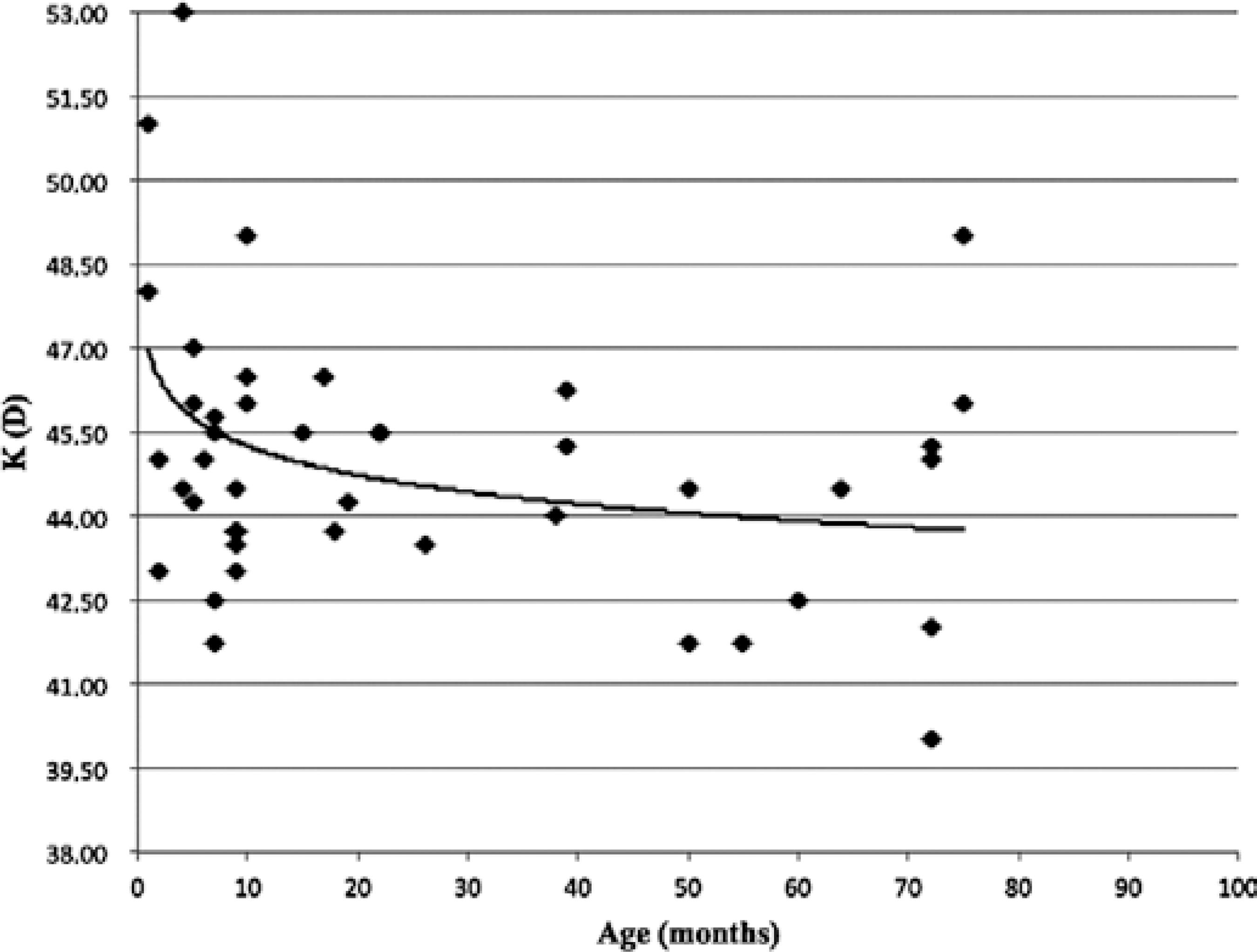
Figure 2 Distribution of the mean keratometry (K) values of 44 eyes of children with unilateral cataracts and randomly selected eyes in the bilateral cases.
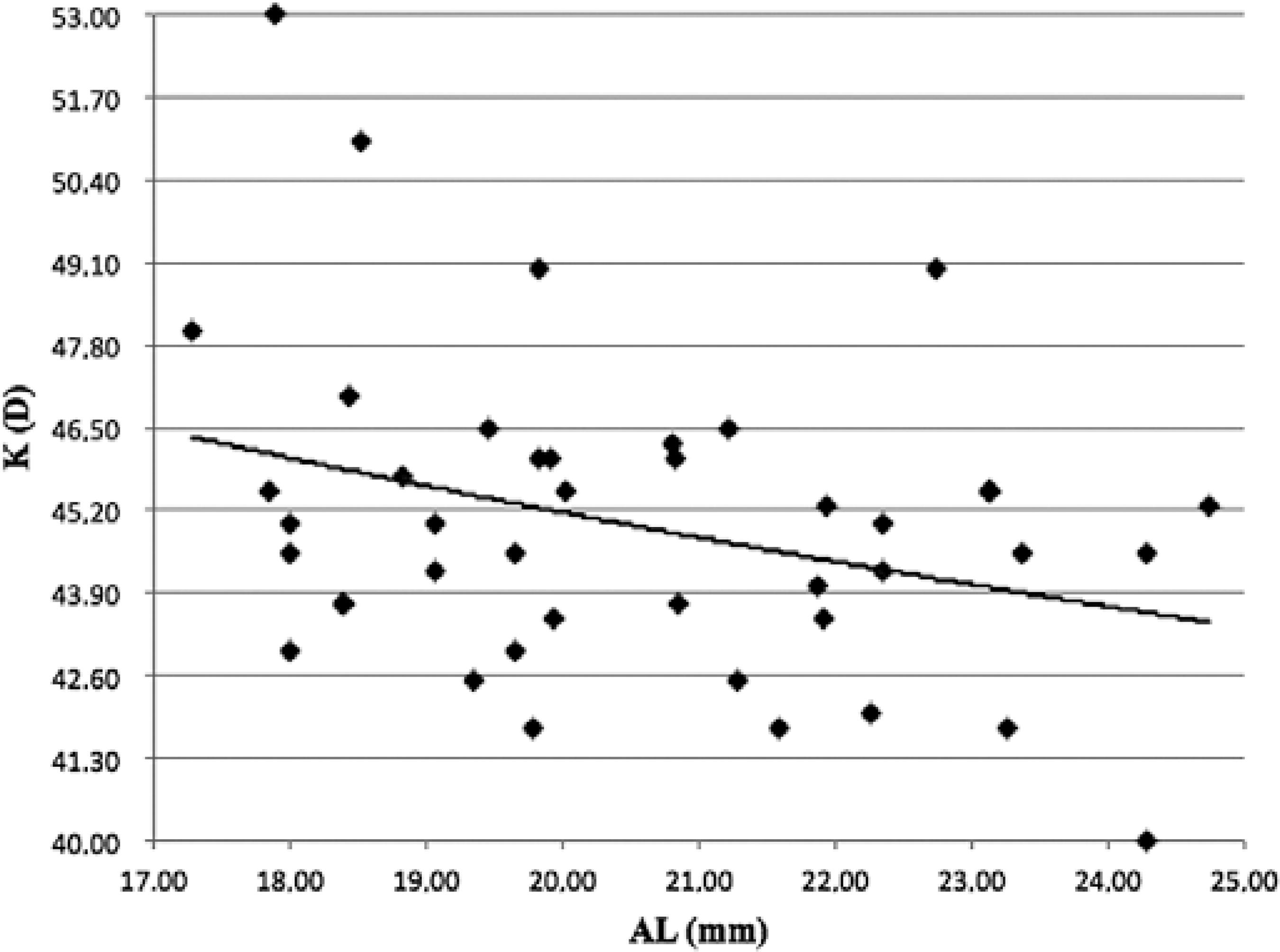
The dispersion values of AL according to K and the inverse relationship between them are shown in figure 3. The equation provided for the graph is K=52.91 -0.385 × AL (mm), withR2=0.11 (P< 0.05).
Table 3 shows a comparison of the biometric measurements between eyes with a unilateral cataract and the corresponding healthy eye, with no significant differences found in the AL and K values (P> 0.05).
Table 3 Comparison of the mean, median, and standard deviation of axial length (AL) and the mean keratometry (K) values of both eyes of 15 children with unilateral cataract
| n | AL (mm) | K (D) | ||
|---|---|---|---|---|
| Cataract eye | 15 | Mean ± SD | 20.6 ± 2.0 | 44.8 ± 2.7 |
| Median | 20.8 | 44.5 | ||
| Range | 17.9-24.7 | 41.7-53.0 | ||
| Healthy eye | 15 | Mean ± SD | 21.3 ± 1.7 | 44.4 ± 1.5 |
| Median | 20.9 | 44.5 | ||
| Range | 18.8-24.8 | 42.0-48.5 | ||
| P value | 0.061 | 0.649 |
AL= axial length; K= keratometry; mm= millimeter; D= diopter; SD= standard deviation. Student's t-test.
Tests of the two regressions for the AL and K logarithm models showed that there were no statistically significant differences between eyes with cataract from different genders, eyes with unilateral cataract and the ones with bilateral cataracts, and normal eyes and eyes with cataract in children with unilateral cataract (P> 0.05).
Table 4 estimates AL and K according to age (in months) based on the following equations: AL=16.66 + (1.476 × ln age) and K=46.97 + (-0.752 × ln age), from figures 1 and 2 and the IOL for emmetropia and for minimizing myopic shift in adulthood, using suggestions from some authors(8), according to age, based on the estimated values of AL and K.
Table 4 Axial length (AL) and keratometry (K) values estimated by age according to the linear regression analysis performed using the natural logarithm (ln) of the patient's age as an independent variable: AL=16.66 + (1.476 x ln age) and K=46.97 + (-0.752 x ln age), as well as the intraocular lens (IOL) power calculated for emmetropia based on the values of K and AL using the Hoffer Q formula with pACD=5.26 (A-constant=118.5). The indicated desired refraction to minimize late myopia shift8 and the ideal IOL power to obtain the desired refraction are also demonstrated
| Age (months) | AL (mm) | K (D) | IOL to emmetropia (D) | Desired refraction (D) | Ideal IOL power for desired refraction (D) |
|---|---|---|---|---|---|
| 3 | 18.28 | 46.14 | +41.36 | +9.00 | +26.90 |
| 6 | 19.30 | 45.62 | +35.73 | +8.00 | +23.11 |
| 9 | 19.90 | 45.32 | +32.95 | +7.00 | +22.06 |
| 12 | 20.33 | 45.10 | +31.14 | +6.00 | +21.93 |
| 18 | 20.93 | 44.80 | +28.81 | +6.00 | +19.53 |
| 24 | 21.35 | 44.58 | +27.31 | +5.00 | +19.67 |
| 30 | 21.68 | 44.41 | +26.18 | +5.00 | + 18.51 |
| 36 | 21.95 | 44.28 | +25.29 | +5.00 | + 17.58 |
| 42 | 22.18 | 44.16 | +24.56 | +5.00 | +16.82 |
| 48 | 22.37 | 44.06 | +23.97 | +4.00 | + 17.87 |
| 54 | 22.55 | 43.97 | +23.42 | +4.00 | + 17.30 |
| 60 | 22.70 | 43.89 | +22.97 | +3.00 | + 18.45 |
| 66 | 22.84 | 43.82 | +22.56 | +3.00 | +18.03 |
| 72 | 22.97 | 43.75 | +22.19 | +2.00 | + 19.21 |
AL= axial length; K= keratometry; mm= millimeter; D= diopter; IOL= intraocular lens.
DISCUSSION
The development of cataract surgery in children has created the need to study the biometric and refractive changes that occur with ocular growth(9,10). In the first months of extra-uterine life, the human eye experiences axial growth accompanied by flattening of the corneal curvature(11,12). When this process occurs uneventfully, the decrease of the refractive power of the lens focuses the image on the retina because of the increased AL. During this rapid biometric change, emmetropia is maintained(13).
Eyes presenting cataract have different biometric measurements from normal eyes, and various factors, such as the child's age at surgery, aphakia, pseudophakia, cataract laterality, and visual deprivation have been reported to influence axial growth(12).
Researchers have reported the existence of a passive ocular-stretching component that is genetically determined and an active component that is observed when the image is not formed on the retina(13). Supporting this hypothesis, several studies have observed increased AL in eyes with visual deprivation(14). This increase occurs in human eyes as well as in the eyes of primates and other animals(6,13).
Several studies have demonstrated flattening of the K values in older children(11,15). A previous study reported mean K values of 47.50 D in newborns and 43.69 D in children aged 2-4 years and concluded that K values reach adulthood values at the age of approximately 3 years(16). In the present study, the eyes with congenital and developmental cataract showed K values that were significantly more curved in children younger than 6 months old. Although it presented a poor correlation (R2=0.14), the value of K was inversely related to the ages of the children up to 18 months. After this age, corneal flattening tends to stabilize.
It has been demonstrated that normal eyes have a smaller myopic shift in comparison to aphakic eyes(11). The reason for this difference is that phakic eyes exhibit a decline in the refractive power of the crystalline lens from +34.4 D to +18.8 D with growth, which does not occur with an implanted IOL(11). Therefore, children with pseudophakia might present a large myopic change in adulthood if the implanted IOL aims for emmetropia at the time of surgery(6).
Two papers have described K and AL measurements in North American children with congenital cataract(5,17). Further, a study in Italy showed the AL and K values of a Caucasian pediatric population with congenital/developmental cataract(7). Databanks containing biometric information from eyes with pediatric cataract allow estimation of the ocular values of AL and K based on age. These data facilitate the selection of IOL power in children with cataract when it is not possible to measure AL and K.
In accordance with the results from another study(18), Brazilian children showed corneal flattening and increased AL with age. In the study by Ingaki(18), there was no significant difference between K and AL in a comparison of eyes with unilateral and bilateral cataracts. The same results were obtained from comparing healthy eyes and affected eyes in children with unilateral cataract. Additionally, in studies of AL and K in North American children, lower AL and greater K were found in eyes with unilateral cataract compared with the AL and K values in healthy eyes, although the difference in the K value was not statistically significant(5,17). As visually impaired eyes tend to have greater axial growth(12), the absence of differences between the AL value of healthy eyes and that of eyes in children with unilateral cataract might have resulted from later diagnosis and treatment in our sample, which could have resulted in worsened visual prognosis for these eyes(19).
Regarding gender, previous studies have reported steeper corneas and shorter AL in girls than in boys; however, these results were not observed in our sample(5,17).
To avoid the development of high myopia with axial growth, some authors recommend that after the calculation of the IOL for emmetropia, 20% of the IOL power should be subtracted in young children (<8 months of age), and 10% should be subtracted in children aged between 2 and 3 years(16). Others propose tables with residual hyperopia from +12.00 to +0.50 based on the age of the children, from 3 months to 14 years(8). After the surgical treatment, optical correction or contact lenses are prescribed for residual hyperopia, which decreases with age. Table 4shows suggestions, based on our data, for IOL implantation according to age for emmetropia and for minimizing late myopia using the suggestions from some authors(8) when AL and K cannot be measured.
When K measurement is not possible because of the unavailability of manual keratometers, refractive powers of 28, 27, 26, 24, and 22 of the implanted IOL are suggested for ALs of 17, 18, 19, 20, and 21 mm, respectively(20). We suggest that when only AL can be measured, the K value should be calculated using the equation: K=52.91-0.385 × AL (mm) (Figure 3) or based on age.
The selection of the power of the IOL for implantation in a growing eye represents a major challenge. The use of a published table alone to decide IOL power is not recommended. The tables are only intended as a starting point toward appropriate IOL power selection, which is a multifactorial decision customized for each child based on many variables, particularly age, laterality, amblyopia status, likely compliance with glasses, and a family history of myopia(8).
The main limitation of this study is the K values. The measurements were performed with a manual keratometer which, although reproducible in awake patients, may not be as reliable in our sample where most of the measurements were performed on patients under anesthesia without fixation. These conditions probably influenced these values and their relationships with age and AL, generating a poor correlation (R2=0.11). These findings have already been described by other authors, who also found weaker relationships between K and age, with R2 varying from 0.31(17) to 0.20(7), as well as between K and AL (R2=0.32)(17), despite the larger sample sizes. Due to the difficulty of obtaining these values, the measurement method used in the present study still seems to be the best manner to obtain K values; however, a device that allows more accurate measurements may establish prediction models with more precise estimates of K in relation to age and AL.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

