INTRODUCTION
Age-related macular degeneration (AMD) is a disease initially characterized by the presence of drusen and abnormal pigmentation of the retinal pigment epithelium (RPE) and later by geographic atrophy, choroidal neovascularization, RPE detachment, and fibrosis. Of the four leading causes of blindness, AMD is the only one for which prophylaxis and treatment remains unclear. This is mainly because of a lack of knowledge with regard to its etiology and pathophysiological mechanisms involved in the different stages of the disease(1). Currently, there are several treatments; however, none of these appear to be sufficiently effective. Therefore, a more extensive study of AMD pathophysiology is necessary to optimize treatment(2-9).
AMD appears to be a multifactorial disease. Genetics is likely to play a key role in its occurrence; furthermore, oxidative stress, ischemia, aging of RPE, and inflammation were other possible etiological factors(10,11). A tyrosine-histidine change at the 402th amino acid position in the complement factor H on the chromosome 1 is strongly associated with AMD(12-14), along with factors such as race, age, and smoking. An inflammatory cycle is also believed to be involved (including immune complex formation, complement activation, extracellular matrix proteolysis, and choroidal T cell and other cell activations), which damages RPE with concomitant degeneration of photoreceptor cells that can extend into more internally located retinal layers.
Although AMD primarily comprises the external layers of the retina, the vitreous may play a role in its etiopathogenesis and/or progression and that inflammation most likely begins in RPE and may even reach the vitreoretinal interface(15). Moreover, some reports have demonstrated a higher rate of vitreoretinal adhesion in AMD(16-22), perhaps contributing to the unfavorable evolution in some cases and/or an insignificant response to intravitreal anti-vascular endothelial growth factor (anti-VEGF).
This study aimed to assess whether hyaloid adhesion is more prevalent in patients with AMD than in control patients and to evaluate whether the prevalence is higher in exudative or non-exudative AMD.
METHODS
This is a cross-sectional analytical study with a control group. Patients were recruited from the Ophthalmology Department of the Public Service Hospital of the State of São Paulo. Patients who received a diagnosis of AMD that was confirmed by fundus biomicroscopy and fluorescein angiography from May 2010 to November 2014 were included. Eyes in all stages of non-exudative (drusen, pigment alterations, and atrophy) and exudative AMD (active or disciform scar) were included. The study was approved by the Ethics Committee of Hospital do Servidor Público do Estado de São Paulo and all patients read and then signed a consent form prior to the initiation of the study.
We excluded all patients presenting with other vitreoretinal afflictions concomitant with AMD and/or previous eye surgery and/or any previous intraocular treatment, such as laser or intravitreal injection and/or previous history of ocular trauma or myopia equal or higher than two diopters.
For optimal study of the vitreoretinal interface, all patients underwent ultrasonography (USG; UltraScan®, Alcon) and spectral-domain optical coherence tomography (SD-OCT) imaging (Cirrus HD-OCT, version 4000; Carl Zeiss Meditec). All USG examinations were performed by the same examiner using the transpalpebral contact technique with a 10-MHz probe and 75-dB gain in modes A and B. SD-OCT was performed by a different examiner following drug-induced mydriasis (10% phenylephrine, 3 drops); however, with respect to the USG examinations, all procedures were performed by the same examiner. We used the 5-line raster scan mode (4,096 A-scans on each of the five lines) and Macular Cube 512 × 128. Only the macular area was evaluated.
Adherence was considered when an average reflectivity line was observed to be partially adherent to the nerve fiber layer. Adhesion was excluded when this line was observed above the nerve fiber layer. When the line was not observed, USG was evaluated to determine whether the hyaloid was anterior or not detached. We considered adherence to be present when the hyaloid was visually adherent to the retinal surface of the macular area in SD-OCT, when it was visually adherent to the macula in USG and SD-OCT, and when the hyaloid was not visible by either method. Non-adherence was considered when the hyaloid was visually non-adherent to the retinal surface in the macular area in the SD-OCT, when it was visually non-adherent to the macula in USG and SD-OCT, whether by total or partial hyaloid detachment (adherent to the optic nerve).
For comparative analysis, we selected patients without vitreoretinal alterations, with similar average ages, and who did not meet the exclusion criteria.
To investigate the relationship between vitreoretinal adhesion and AMD, we performed a non-parametric Fisher's exact test considering two forms of the disease: exudative (active membrane or disciform scar) and non-exudative AMD (drusen or geographic atrophy), with a third group serving as the control. Age uniformity was verified by analysis of variance (F=1.959, p=0.131). Results with p values of ≤0.05 were considered statistically significant.
RESULTS
We assessed 75 eyes of 23 patients with AMD (14 women and nine men) and 15 in control group (11 women and four men). In total, 33 eyes had AMD that was consistent with the inclusion criteria; of which 11 had the non-exudative form (non-atrophic) and 22 had the exudative form (11 active and 11 disciform scars).
In the AMD group, we observed two patients with the non-exudative form in one eye each and the disciform scar in the contralateral eye, one patient with disciform scars in both eyes, three patients with the non-exudative form in one eye each and a choroidal neovascular membrane (CNVM) in the other eye, five patients with disciform scars in one eye each and CNVM in the other eye, one patient with the non-exudative form in both eyes, and one patient with CNVM in both eyes.
Twelve eyes were excluded from the study for the following reasons: Ten were pseudophakic (three disciform, one atrophic, and six active CNVM) and two had already received an anti-VEGF injection. One was excluded for having received intravitreal injections (active form) and another for undergoing laser treatment (disciform scar).
The average age of the patients was similar across the groups (Table 1).
Table 1 Average age, standard deviation, and 95% confidence intervals for patients in each study group. Analysis of variance (F=1.959, p=0.131) was used for verification, and no difference in age was observed the three groups
| Group | Average ± standard deviation | 95% confidence interval |
|---|---|---|
| Control | 69.64 ± 6.19 | 67.24-72.04 |
| Dry AMD1 | 77.18 ± 9.61 | 70.72-83.64 |
| Exudative AMD | 72.45 ± 11.64 | 67.01-77.90 |
1AMD= age-related macular degeneration.
All eyes were assessed by SD-OCT and USG in a complementary manner, and adhesion was found in 7/22 (31.82%) eyes with exudative AMD, in 5/11 (45.45%) eyes with non-exudative AMD, and in 8/30 (26.67%) eyes in the control group. Non-adhesion was diagnosed in 15/22 (68.18%) eyes with exudative AMD, in 6/11 (54.55%) eyes with non-exudative AMD, and in 22/30 (73.33%) eyes within the control group (Table 2). While separately evaluating cases of exudative AMD, we found adhesion in 6/22 (27.27%) eyes with active neovascular membranes and in 1/22 (4.54%) eyes with disciform scars. Adhesion was absent in 5/22 (22.72%) eyes with active neovascular membranes and in 10/22 (45.45%) eyes with disciform scars.
Table 2 Comparison1 of vitreoretinal adhesion rates between age-related macular degeneration (AMD) and control groups
| Vitreoretinal adhesion | |||
|---|---|---|---|
| Group | Adhesion | No adhesion | Total |
| Control | 8 (26.67%) | 22 (73.33%) | 30 |
| Exudative AMD (p=0.762) | 7 (31.82%) | 15 (68.18%) | 22 |
| Dry AMD (p=0.280) | 5 (45.45%) | 6 (54.55%) | 11 |
| Total | 20 | 43 | 63 |
1Absolute frequency and percentage within each group [control, non-exudative age-related (dry) macular degeneration and exudative age-related macular degeneration] related to vitreoretinal adhesion, evaluated by spectral domain optical coherence tomography and ultrasonography.
In six patients with AMD, SD-OCT revealed vitreoretinal adhesion (Figure 1), whereas USG detected two cases of partial posterior vitreous detachment (PVD), with both being adherent to the macula, as shown in figure 2 (in four cases, there was no PVD). In the 25 cases of AMD in which the hyaloid was not visible by SD-OCT (Figure 3), USG detected total PVD in five eyes, mobile vitreous membranes in six eyes, and partial PVD in 14 eyes (adherent to the optic nerve). In one of the two cases in which SD-OCT revealed total detachment of the hyaloid in the macular area, while USG revealed no detachment.
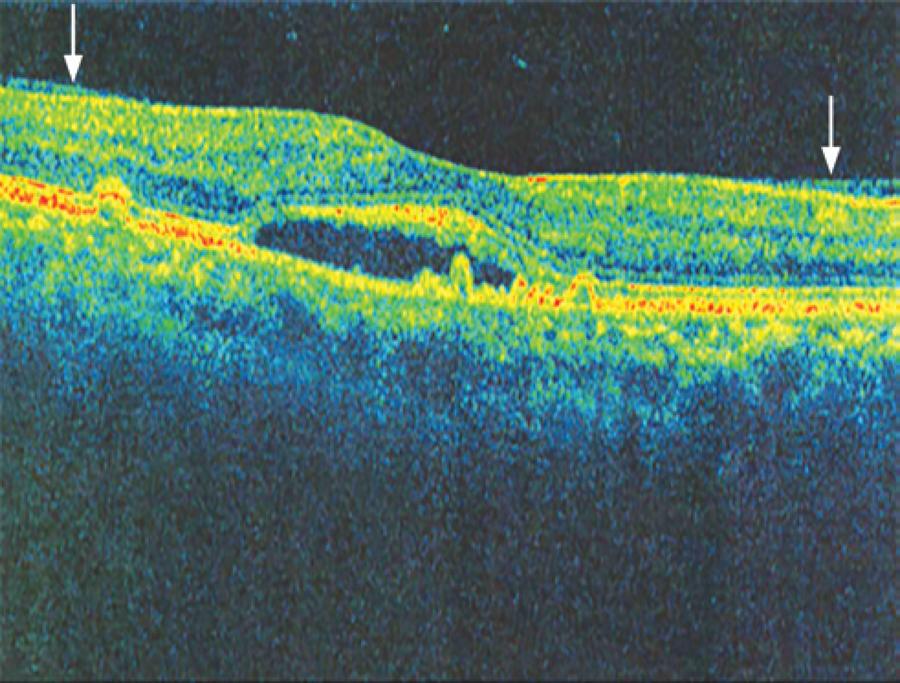
Figure 1 Image obtained with spectral domain optical coherence tomography (Cirrus HD-OCT), showing the hyaloid adhering to the macular area (arrows).
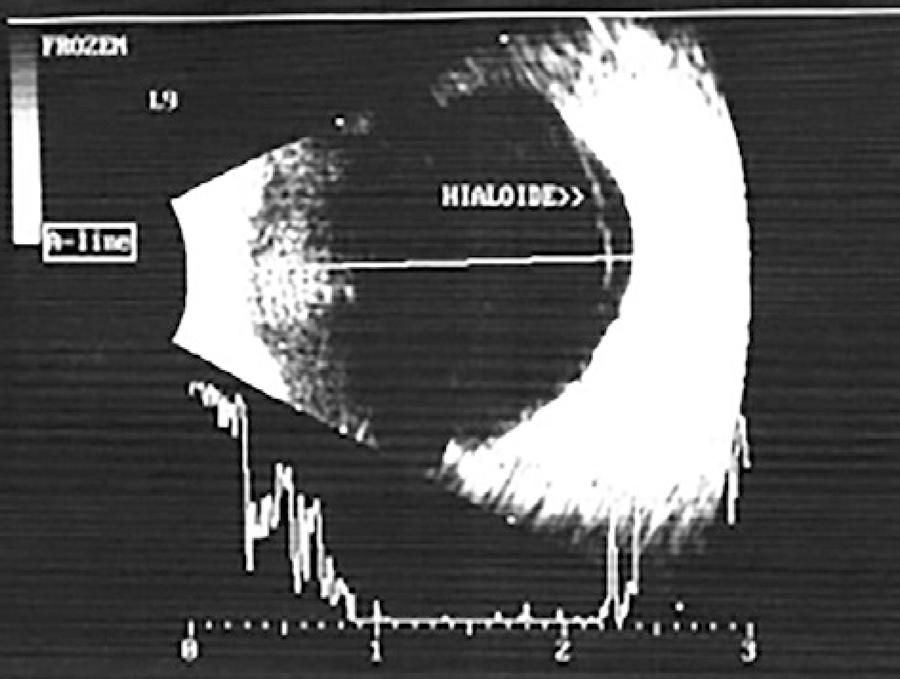
Figure 2 Image obtained by ultrasonography using a 10-MHz transducer. The hyaloid adhering to the neovascular membrane is visible.
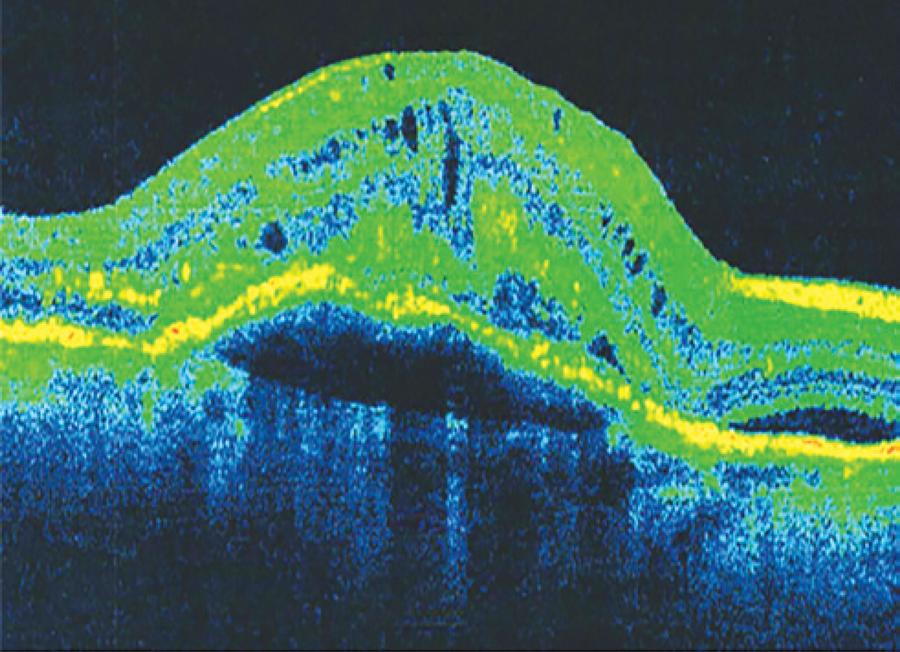
Figure 3 Image obtained by spectral domain optical coherence tomography (Cirrus HD-OCT). The hyaloid is not evident.
USG detected mobile vitreous membranes in all nine cases in the control group that had hyaloid adhesion in the macula on SD-OCT examination. In 19 cases in which the hyaloid was not visualized in SD-OCT, USG detected eight cases of partial PVD (adherent to the optic nerve), eight cases of total PVD, and three cases with mobile vitreous membranes. In the two cases in which the hyaloid was non-adherent, USG revealed partial PVD in one case (adherent to the optical nerve) and total PVD in the other case.
Moreover, vitreoretinal adhesion was not associated with AMD presence. The proportion of patients with and without adhesion who were evaluated by SD-OCT and USG did not differ between the AMD (non-exudative and exudative) and control groups (X2=0.682; p=0.432). Thus, given the study conditions, vitreoretinal adhesion was neither higher nor lower in patients with AMD than in control patients.
The proportion of patients with and without adhesion who were evaluated by SD-OCT and USG did not differ between the non-exudative and exudative AMD groups (X2=0.589; p=0.471). Thus, given the study conditions, vitreoretinal adhesion was neither higher nor lower in patients with the exudative form than in those with the non-exudative form.
The proportion of patients with and without adhesion who were evaluated by OCT and US did not differ between the non-exudative AMD and control groups (X2=1.312, p=0.280) or between the exudative AMD and control groups (X2=0.164, p=0.762).
DISCUSSION
There is still much speculation regarding the role of hyaloid in AMD. The vitreous is an important component in the pathophysiology of several retinal diseases, and hyaloid adhesion is a poor prognostic factor in some. Consequently, questions have been raised regarding the supposed role of hyaloid adhesion in AMD, with several theories being postulated. On the one hand, hyaloid adhesion has been only considered as a risk factor of AMD progression, whereas on the other hand, it has been only considered to be part of its pathophysiology. In other studies, it has been considered as a poor prognostic factor, predisposing either to the development of the exudative form or to a worse response to treatment.
Some studies suggest that hyaloid adhesion may induce a mild chronic retinal inflammation(18,19). This is then posited to hinder oxygen penetration and to cause chronic ischemia or VEGF retention in the macular area. Furthermore, hyaloid traction may lead to RPE disruption, which is known to induce the appearance of the neovascular membrane. Embryological, molecular, and structural similarities have been demonstrated between Bruch's membrane and the internal limiting membrane, thus supporting the theory that hyaloid may play a role in AMD(23).
We assessed the presence of vitreoretinal adhesion in patients with AMD to test its prevalence against that of a control group. When the hyaloid detachment develops close to the retina, the presence of vitreoretinal adhesion or traction can be difficult to diagnose using USG; therefore, we used SD-OCT for optimal assessment. However, SD-OCT images in cases with either total hyaloid adhesion in the macular area or total detachment with hyaloid anteriorization are similar (the hyaloid is invisible); thus, the use of USG becomes indispensable.
Furthermore, we chose not to distinguish between adhesion and traction because it has already been performed in some studies. Therefore, the objective was limited to assessing whether the prevalence of adherent hyaloid was higher in patients with AMD than in patients of the same age without AMD because it is known that the incidence of PVD increases with age.
In this analysis, we did not observe a higher vitreoretinal adhesion in patients with AMD than in the control patients, and we did not determine higher adhesion rates in patients with the exudative form than in those with the non-exudative form.
However, the evidence for a higher prevalence of vitreous adhesion in AMD remains conflicting. Some studies demonstrated higher hyaloid adhesion at all stages of AMD(17,19,20), whereas others demonstrated higher adhesion rates in the initial phases only(18).
Vitreomacular adhesion was assessed in patients with AMD(17) by dividing patients into exudative AMD, non-exudative AMD, and control groups. The presence of hyaloid adhesion in the macular area was analyzed by OCT/confocal scanning laser ophthalmoscopy, whereas the presence of total PVD was evaluated by fundus biomicroscopy. Adhesion in patients with AMD was higher than in control patients; however, there was no difference between patients with exudative AMD and those with non-exudative AMD.
In another study(20), patients were divided in the same manner; however, only the presence of total PVD was assessed with USG. This approach also led to identifying a higher percentage of total PVD in the control group; however, no difference was observed when non-exudative and exudative AMD were compared.
In another analysis(24), when adhesion was assessed by SD-OCT (Spectralis OCTTM; Heidelberg Engineering, Heidelberg, Germany) and fundus biomicroscopy in patients with active exudative AMD only, the result was different because of the higher rate of non-adhesion within the study group.
Another study(25)evaluated cases of non-exudative AMD that were classified as high risk (i.e., category IV according to the Age-Related Disease Study) using patients with a CNVM in one eye and non-exudative high-risk AMD in the contralateral eye. Hyaloid adhesion was assessed by the time-domain OCT (OCT3, Carl Zeiss, Meditech, Dublin, CA), SD-OCT (Cirrus Version, Carl Zeiss) and the presence of the Weiss ring on biomicroscopy examination, thus concluding that there was no significant influence of vitreomacular adhesion on the development of the exudative form.
Furthermore, another study with a similar design(22) assessed hyaloid adhesion by SD-OCT (Stratus OCT3, Zeiss Humphrey, San Leandro, CA) in patients with CNVM in one eye and no signs of CNVM or drusen in the contralateral eye. The authors concluded that vitreoretinal adhesion might be a risk factor for exudative AMD because most eyes with adhesion exhibited active exudative AMD.
In another study(19), patients were divided in a similar manner as in previous studies(17,20) but with the control group comprising contralateral eyes, as detailed elsewhere (22,25). Using USG and SD-OCT (Stratus III, Carl Zeiss, San Leandro, California, USA) to verify the status of the hyaloid, the authors discovered higher adhesion rates in patients with exudative AMD than in controls and patients with non-exudative AMD.
In an alternative assessment of hyaloid adhesion in AMD(18), two groups were formed; one with druse in one eye and CNVM in the contralateral eye and another with atrophy in one eye and disciform disease in the contralateral eye. The presence of vitreomacular adhesion was evaluated by SD-OCT (OCT-SLO, OCT1000, and Stratus III OCT), and the presence of PVD was evaluated by USG. Similar to a previous study(22), it was concluded that total PVD might be protective against CNVM, whereas adhesion may be a risk factor for CNVM.
Further, in an evaluation of cases of CNVM using SD-OCT (OCT3, Carl Zeiss)(21), it was reported that most cases presented with abnormalities in the vitreoretinal interface.
When analyzing the existing studies, one will notice contradictory and inconclusive results, with many different methodologies, apparatuses, and groups being analyzed.
Given the results of this study, we believe that the hyaloid does not have a significant role in AMD pathophysiology. However, in cases of adhesion and CNVM, we observed that the hyaloid was always adherent in the membrane area, suggesting that a relationship could indeed exist between CNVM and hyaloid adhesion, as demonstrated in other studies(15,18,19,21,22).
A possible explanation is that a more intense retinal inflammation could be present in some cases of exudative AMD.
Nevertheless, questions remain as to whether such an inflammation would be a cause or consequence. Similarities in molecular composition and structural organization of the vitreoretinal interface and between the retina and RPE suggest that both interfaces may be subject to the same aging processes and that abnormalities of the first may alter or amplify the degenerative processes of the latter and vice-versa(23).
In this study, AMD stages (CNVM vs. disciform) were not separately evaluated; however, the percentage of adhesion in eyes with CNVM (27.7%) was higher than in eyes with disciform disease (4.54%). We failed to determine higher adhesion rates in the exudative group because of perhaps the high incidence of non-adhesion in the disciform group (10/22).
Moreover, it is possible that hyaloid detachment is a natural process in AMD evolution, such as atrophy and disciform scar formation, which develops once the appearance of Muller cells would affect the integrity of the internal limiting membrane, thereby promoting hyaloid detachment. This hypothesis is consistent with previous observations(18) and with our finding that adhesion occurred in 27.7% of the eyes with CNVM and in only 4.54% of those with disciform disease. However, further discussion is beyond the scope of this paper.
As demonstrated by a few studies, the hyaloid may only act as a poor prognostic factor for improving visual acuity following treatment, particularly when there is a traction in the macular area(24,26-28). In those cases, there is a disruption of the retinal layers that was caused not only by CNVM but also by hyaloid traction, thus leading to a worse visual prognosis. Alternatively, if the hypothesis of inflammation equally developing in the vitreoretinal interface and between the retina and RPE is confirmed, it would be conceivable that more severe degrees of inflammation result in both CNVM and in adhesion/traction/epiretinal membranes, as observed in a study(21). To improve visual prognosis of those cases, removal of the hyaloid may be justifiable.
We believe that despite the limited sample size of this study, the role of the hyaloid remains questionable. Further research must continue with greater degrees of standardization in the groups evaluated and in the methods used.
It is important to pay special attention to the study of the hyaloid in cases with high-risk non-exudative AMD, as performed by a previous study(25), particularly before recommending vitrectomy as a preventive measure against CNVM or macular atrophy(29).
Thus, it must be evaluated whether performing vitrectomy would adequately treat AMD. Finally, it is necessary to clarify whether removing the vitreous would reduce the incidence of AMD or just that of CNVM or if it would affect visual prognosis.
CONCLUSION
In this study, when assessed by SD-OCT and USG, patients with exudative and non-exudative forms of AMD did not present with higher vitreoretinal adhesion than control patients without AMD. Moreover, exudative AMD (CNVM and disciform) was not associated with higher levels of adhesion when compared with non-exudative AMD.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

