INTRODUCTION
As life expectancy increases worldwide (about 600 million people are aged 60 years and older)(1), involutional and chronic eye diseases are becoming increasingly important in the spectrum of ophthalmological diseases. The involutional changes that result in eyelid pathology include ectropion and entropion, dermatochalasis, and aponeurotic ptosis.
The advanced loss of laxity and tone, which is the characterizing feature of the aging of ocular adnexal tissue, results in sagging eyelids(2-5). Genetic causation, which cannot be modified by human behavior, is a part of the intrinsic aging mechanism(6,7). However, extrinsic aging can be caused by parameters, such as alcohol use, chronic exposure to sunlight, smoking, and nutrition(6,7).
The only treatment option for involutional eyelid malposition is surgical adjustment of the underlying anatomical involutional disorder. This review underlines the current pathophysiology and clinical management.
Epidemiology
Dermatochalasis, ptosis, ectropion, and entropion are common disorders in middle-aged and older adults. The overall prevalence of sagging eyelids among individuals aged ≧ 45 years is reported to be 16%, comprising 19% of men and 14% of women(8). Two large epidemiologic studies - the Rotterdam study and the Twins UKstudy-dealt with the prevalence and risk factors for sagging eyelids(9). Two large study populations were chosen for these studies: The Rotterdam studyincluded 5578 individuals of North European origin with an average age of 67 years living in the periphery of Rotterdam and the UK twins study involved 2186 twins with an average age of 53 years living in Great Britain(8). By studying these populations, many non-genetic risk factors for sagging eyelids could be discerned, including age, high body mass index, lighter skin color, smoking, male gender, and heritability(8). Genetic analysis showed the C allele of rs11876749 on chromosome 18 to be a defensive risk factor for sagging eyelids(8).
Sufficient data are not yet available regarding the incidence of ptosis(9,10). The most prominent underlying reason for acquired ptosis is aponeurosis(11).
The prevalence of involutional ectropion seems to be similar tothat of involutional entropion(12). The literature suggests that the prevalence of ectropion is about 4% in patients older than 49 years, not differing among the different ectropion forms or regarding the underlying etiology(12,13). This involutional malformation is the most common form of ectropion and entropion(14). Males are more often affected by involutional ectropion(12). In contrast, involutional entropion is more often seen in women(12). This gender difference is caused by the difference in tarsal plate size and axial ocular globe projection between the two genders(12,15,16). The involutional forms of ectropion and entropion affect whites more often than blacks(17,18). The prevalence of involutional ectropion and entropion increases with the patient's age(12), which is the logical order for involutional malformations to increase with age(13).
Pathogenesis and pathological findings
The advanced loss of laxity and tone, which is a characteristic feature of aging ocular adnexal tissue, results in sagging eyelids(2-5). Histologic examination of dermatochalasis specimens shows an increased number and dilation of lymphatic vessels in conjunction with widely spaced collagen bundles. A reduction in elastic fibers, which are essential components of the function and structure of the lymphatic system, has also been demonstrated(5,19). The pathogenesis of dermatochalasis may begin with subclinical inflammation and lead to elastolysis and secondary lymphostasis(5,9).
The most common pathogenesis for ptosis is aponeurotic ptosis(12). The levator muscle starts thinning and begins to lose muscle tone. As a result, it is not able to keep the upper lid in the correct position above the eye and ptosis ensues(9). Disinsertion or dehiscence of the levator aponeurosis can also cause ptosis(9). Other risk factors for ptosis include intraocular surgery and chronic inflammatory diseases that disinsert the levator aponeurosis from the tarsal plate(9). In conclusion, patients wearing hard contact lens or suffering from eye infections and those having undergone ocular surgery or (peri-)ocular trauma are more likely to develop involutional ptosis(9 ). Considering the underlying pathogenesis of age-related aponeurotic blepharoptosis, a recent histopathological study revealed that oxidative stress of the levator aponeurosis also plays a potential role in its pathogenesis( 20).
The lateral laxity of the canthal tendon is the most important reason for the appearance of involutional ectropion and entropion. Laxity of the medial canthal tendon as well as false insertion of the lower retractors are also conductive parameters(12,21-26). Age-related decrease and atrophy of the orbital fat as well as the relaxation of the ligamentous support lead to an increasing eyelid-globe disparity that again compounds eyelid laxity(9,16).
Some studies reported the presence of abnormal elastic fibers in involutional ectropion and entropion; however, they did not measure them nor did they address the possible reasons for the abnormal fibers(21,22). Damasceno et al. revealed a decrease in elastic fibers in the pretarsal orbicularis oculi muscle, in the tarsal stroma, and in the eyelid skin in patients suffering from age-related involutional ectropion and entropion(27). Ultrastructural abnormalities of the elastic fibers were also observed. These findings have been documented in other diseases as well, such as cutis laxa, Ehlers-Danlos syndrome, floppy eyelid syndrome, Marfan syndrome, Menkes' syndrome, progressive systemic sclerosis, and pseudoxanthoma elasticum(28-30). Ultrastructural changes in the elastic fibers of the eyelid in patients suffering from involutional ectropion and entropion may result from a primary defect in the formation of elastin or from the secondary enzymatic degradation of properly formed fibers(27,30). Damasceno et al. have demonstrated an overexpression of elastolytic enzymes, such as MMP-9, MMP-7, and MMP-2(27,30). It remains speculative to reflect whether raised elastolytic activity can result in decreased numbers and structural abnormalities of elastic fibers(30). However, raised elastolytic activity is already known to be responsible for the loss of elastic fibers of the skin in the acquired form of cutis laxa(29,30). MMP-2, one of the most powerful elastolytic enzymes, is released during ischemia and inflammatory processes(30-32). MMP-7 and MMP-9 are responsible for the degradation of the elastic fibers in anetoderma, floppy eyelid syndrome, and mid-dermal elastosis(30,33,34). These elastin-degrading enzymes are released during ischemia, inflammation, and chronic mechanical irritation(30,35-37). Overexpression of elastin-degrading enzymes in involutional ectropion and entropion specimens may be the consequence of local ischemia, inflammation, and/or chronic mechanical stress(27). Increased expression of MMP-2, MMP-7, and MMP-9 is also observed in areas with and without inflammatory cell infiltration and may be induced by a local ischemic lesion, inflammation, and/or repeated mechanical trauma(27,38). Atherosclerosis of the eyelid marginal artery, reported in patients with involutional ectropion(21), chronic blepharitis, and eye rubbing, is an example of such predisposing factors(27). Additionally, upregulation of MMP-7 and MMP-9 by mechanical stress has been demonstrated(30,35,37). In involutional ectropion and entropion, there is a significant negative correlation between horizontal eyelid laxity and extracellular matrix components, including collagen and elastic fibers, so that both components are affected by degeneration (Figure 1)(21,22,39-41). Aging skin is characterized by increasing laxity and decreasing numbers of collagen and elastic fibers(39,42-44). Collagen and elastic fibers are essential components of the extracellular matrix of the eyelid and are responsible for its function(6,7,39). Collagen fibers are liable for the tensile strength; elastic fibers responsible for the flexibility and resiliency(6,7,39).
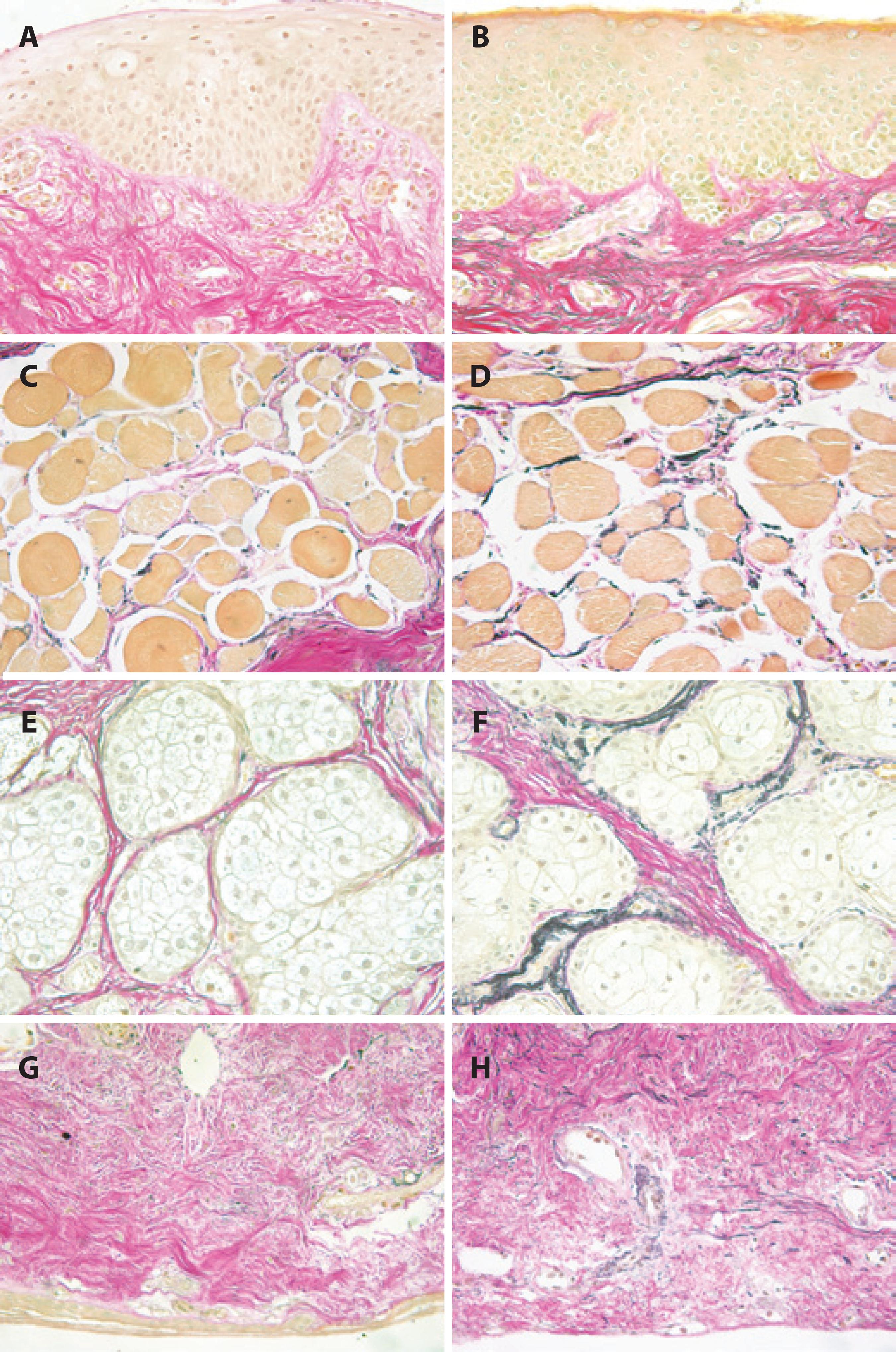
Figure 1 Histopathologic sections of full-thickness eyelid specimens obtained from the lateral lower eyelid of a 65-year-old male patient with involutional ectropion and lateral canthal tendon laxity (A, C, E, G) and controls (B, D, F, H) stained using van Gieson's method for elastic fibers. Elastic fibers appear dark-brown, collagen fibers appear red (Original magnification, 400×). A, B, C, D, Anterior lamellar regions with eyelid skin and pretarsal orbicularis oculi muscle show a nearly complete absence of elastic fibers in involutional ectropion (A, C) compared with control tissue (B, D). E, F, G, H, Posterior lamellar regions with perimeibomian tarsal stroma and intermeibomian tarsal stroma reveal a marked reduction of elastic fibers in involutional ectropion (E, G) compared with control tissue (F, H). (Modified from Reference 42).
In general, two different mechanisms are important for skin aging: the intrinsic and extrinsic systems(6). Genetic causation, which cannot be modified by human behavior, is part of the intrinsic aging mechanism(6,7). Conversely, extrinsic aging can be altered by parameters like alcohol use, chronic exposure to sunlight, smoking, and nutrition deficit(6,7). The most important parameter contributing to 80% of skin aging skin aging appears to be sun exposure(45). Long-term exposure to extrinsic aging parameters or voluntary exposure within a period of time can also cause preterm skin aging(45).
Clinical findings
The pathological processes of eyelid aging with advanced loss of laxity and tone may affect the ocular surface and adnexal tissues, resulting in different clinical symptoms and signs.
Dermatochalasis is an excess of skin weighed down by the effect of gravity. Patients with upper eyelid dermatochalasis often suffer from drooping eyelids, blurred vision, increased tearing or reduced peripheral vision in the upper hemisphere, as well as tired or sleepy appearing eyes(9). In addition to simple folds of loose skin, there is usually a presence of underlying orbital fat, which is often restricted to the medial fat pad in the upper lid(46). A pseudoptosis may be induced by the increased weight of the tissue, but a true ptosis may also be present(46). Both the upper eyelid and, rarely, the lower eyelid can be involved in dermatochalasis. Long-standing massive dermatochalasis of the lower eyelid may lead to ectropion; however, despite all these possible functional symptoms, most of the patients will be more bothered by the cosmetic appearance of the eyelids.
A moderate ptosis may cause symptoms similar to those of upper eyelid dermatochalasis. Severe ptosis decreases the visual field so that patients need to raise their eyebrows or eyelids with their fingers or tilt their heads back at the neck to see from under the sagging eyelids(9). Only these maneuvers will make everyday activities, such as driving, climbing stairs, or reading possible(9). Side effects of raising the eyebrow include headache, painful eyes, and an odd “surprised” appearance(9). The evaluation of levator function allows the correct classification of ptosis severity, which is the basis for the selection of the therapeutic option(47). Levator function can be determined by measuring the amplitude of the upper lid from extreme down gaze to extreme up gaze(47). Using this measure, levator function can be classified as (1) good levator function, when the excursion amounts to 8 mm, (2) moderate, when the excursion amounts to 5-7 mm, and (3) poor, when the excursion amounts 4 mm(47). Typical features of aponeurotic blepharoptosis include good levator function, deep upper lid sulcus, and excessive skin wrinkles(2). Blepharoptosis gets worse in the evening as a result of tired Muller's muscles which antagonize ptosis throughout the day(2,46).
Ocular surface and eyelid abnormalities associated with involution ectropion include lateral canthal tendon laxity (with an incidence of 80%), dry eye (52%), chronic blepharitis (43%), chronic conjunctivitis (40%), lower retractor laxity (40%), superficial punctate keratopathy (29%), and medial canthal tendon laxity (18%).(18) The ocular surface and pathologic eyelid findings associated with involutional entropion include lateral canthal tendon laxity (78%), dry eye (72%), superficial punctate keratopathy (62%), lower retractor laxity (53%), chronic blepharitis (49%), chronic conjunctivitis (23%), and medial canthal tendon laxity (15%)(12). Patients with involutional ectropion more often suffer from chronic conjunctivitis(12) and have a higher axial ocular globe projection(12,16). However, dry eye syndrome, superficial punctate keratopathy, and laxity of the lower retractor are more common in patients with involutional entropion(12,16). No differences can be seen between ectropion and entropion regarding the frequency of chronic blepharitis, lateral or medial canthal tendon laxity(21,24,26). The most common etiologic factor for involutional ectropion and entropion of the lower eyelid is lateral canthal tendon laxity(24-27,29).
Surgical management
Because of the anatomical conditions and pathogenesis of involutional eyelid malposition, favorable surgical management is possible(3,10,47). Dermatochalasis can be corrected through upper and lower eyelid blepharoplasty. After marking the area of excessive skin, the surgeon begins the upper blepharoplasty with an incision on the lid crease and finishes with an incision along the marked area of excessive skin. The surgeon should prepare and separate a flap including both skin an orbicularis muscle(48) to avoid excessive bleeding. A new lid crease can be created by suturing the levator aponeurosis to the orbicularis muscle(48).
Involutional ectropion can be treated by shortening or tightening the underlying anatomical structures(47). A modified Bick procedure can be performed when the ectropion is located centrally. This procedure requires a pentagonal excision of full thickness, followed by direct closure 6 mm in from the lateral canthus(47). A lateral tarsal strip procedure can be performed for a laterally located ectropion(47). The nasolacrimal system needs special protection during functional reposition of a medial ectropion(47).
Surgical methods for treating entropion incorporate transverse lid split, horizontal shortening, everting sutures, and retractor application(47).
Surgical treatment of aponeurotic ptosis depends on levator function(47). To treat the disinsertion of the levator aponeurosis, an aponeurosis advancement is usually performed while good levator function still exists(47).




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

