INTRODUCTION
Inflammatory bowel disease (IBD) represents a group of inflammatory conditions affecting the colon and small intestine that primarily includes Crohn's disease (CD) and ulcerative colitis (UC). Although intestinal inflammation is the primary process underlying IBD pathology, extraintestinal manifestations (EIMs) are reportedly observed in 5%-40% of patients with IBD and represent the first indication of disease in 10%-20% of cases(1).
Ocular inflammation in IBD was first reported by Crohn in 1925 with a reported prevalence ranging from 4% to 30%(2,3). Episcleritis, scleritis, and uveitis are by far the commonest ophthalmic manifestations of IBD, with uveitis the most frequently observed, as patients typically delay treatment for episcleritis that typically has a mild, self limiting course(4). Uveitis has been reported in up to 17% of patients with IBD and 2% of uveitis patients reportedly have IBD(4-6). The course of the uveitis may not parallel IBD activity. The prompt diagnosis of uveitis and therapy with topical or systemic steroids is necessary to avoid severe complications.
Spectral domain optical coherence tomography (SD-OCT) is a noninvasive method for studying retinal layer structures in a range of retinal conditions(7). Recently, a new method of SD-OCT, termed enhanced depth imaging (EDI), has been shown to be capable of visualizing the full thickness of the choroid(8). Changes in choroidal thickness have previously been reported in a number of autoimmune and systemic inflammatory disorders(9-13). To the best of our knowledge, studies using EDI-OCT to evaluate choroidal segment involvement in patients with IBD have yet to be reported.
In this study, we evaluated the efficacy of increased choroidal thickness as a marker of disease activity in patients with IBD.
METHODS
We performed a cross-sectional study involving EDI-OCT analysis of 62 eyes of 31 IBD patients (CD, n=10; UC, n=21; mean age, 44.6 ± 1 3.9years; 11 males; 20 females) and 104 eyes of 52 healthy volunteers (mean age, 44.7 ± 12.6 years; 32 males; 20 females). All patients were sequentially recruited into this study. Clinical and laboratory characteristics of IBD patients are presented in table 1 and table 2. Crohn's disease activity was assessed using the Crohn's disease activity index (CDAI). UC disease activity was assessed using the Truelove-Witts score and Lichtiger index(14-16). The Rachmilewitz endoscopic index was used to evaluate endoscopic activity in UC patients(17). Exclusion criteria included spherical equivalent values greater than ± 4.0 diopters, coexisting ocular disease such as glaucoma or ocular hypertension, and any previous history of ophthalmic surgery, ocular trauma, retinopathy, optic neuropathy or other neurological and systemic vascular disease. The present study was approved by the Gazi University Ethics Committee (26/01/2015 Nº 53). Informed consent was obtained from all participants prior to examinations.
Table 1 Clinical characteristics of inflammatory bowel disease (IBD) patients
| IBD n=31 | |
|---|---|
| Age, years (mean±SD) | 44.6 ± 13.9 |
| Sex, male/female | 11/20 |
| Ulcerative colitis, n (%) | 20 (65) |
| Crohn's disease, n (%) | 11 (35) |
| Disease duration, years, median (min-max) | 4 (0.5-29) |
| Smoking, n (%) | 15 (48.0) |
| Appendectomy, n (%) | 3 ( 9.7) |
| IBD-related surgery, n (%) | 1 ( 3.2) |
| Family history of IBD n (%) | 3 ( 9.7) |
| Medical therapy n (%) | |
| 5-ASA | 29 (93.5) |
| Aza | 10 (32.2) |
| Steroids | 10 (32.2) |
| Anti-TNFα | 2 ( 3.3) |
| Medical istory of steroid therapy, n (%) | 20 (65.0) |
ASA= aminosalycilic acid;
Aza= azathiopurine;
TNFα= transforming growth factor α.
Table 2 Laboratory characteristics of inflammatory bowel disease (IBD) patients
| IBD n=31 | |
|---|---|
| Hemoglobin (g/dl) | 12.7 ± 1.9 |
| Hematocrit | 39.0 ± 5.6 |
| Platelet count (/mm3) | 299969 ± 98175.9 |
| Serum C-reactive protein level (mg/L) | 9.8 ± 9.4 |
| Eryhtrocyte sedimentation rate (mm/hr) | 17.9 ± 13.1 |
Numerical values represent mean ± SD. Normal ranges= Hb (male= 13.5-17.5; female= 12-16), hematocrit (male= 41-53; female= 36-48), platelet counts (150000/mm3-40000/mm3), serum C-reactive protein level (0 mg/L-6 mg/L), and erythrocyte sedimentation rate (0 mm/hr-20 mm/hr).
Spherical equivalent refractive error, best corrected visual acuity (BCVA), ocular pressure, slit-lamp biomicroscopy, fundus examination, and ocular inflammation scores based on summed scores of the presence of cells and flares in the anterior chamber and vitreous were evaluated in all patients(18). All participants in the control group had a BCVA equal to 1.0 and no clinical evidence of retinal disease or IBD. The EDI mode of an SD-OCT (Spectralis HRA+OCT; Heidelberg Engineering Inc, Heidelberg, Germany) was used to evaluate choroidal thickness with all measurements obtained by the same operator during morning hours. Twenty-five sections composed of 40 averaged scans were obtained within a 10°×20° rectangle centered on the fovea. Two scans, a vertical and a horizontal scan through the centre of the fovea, were selected and choroidal thickness was measured from the outer edge of the retinal pigment epithelium to the inner edge of the suprachoroidal space below the fovea. Foveal scans on the same eye at different time points were conducted under identical scanning conditions automatically retrieved from the follow-up mode of the EDI-OCT system. Measurements were made of the subfoveal choroid and at 3000 mm nasal and 3000 mm temporal to the center of the fovea (Figure 1). Choroidal thickness was measured from OCT data from horizontal sections under the center of the fovea by two blinded, independent observers and averaged for analysis.
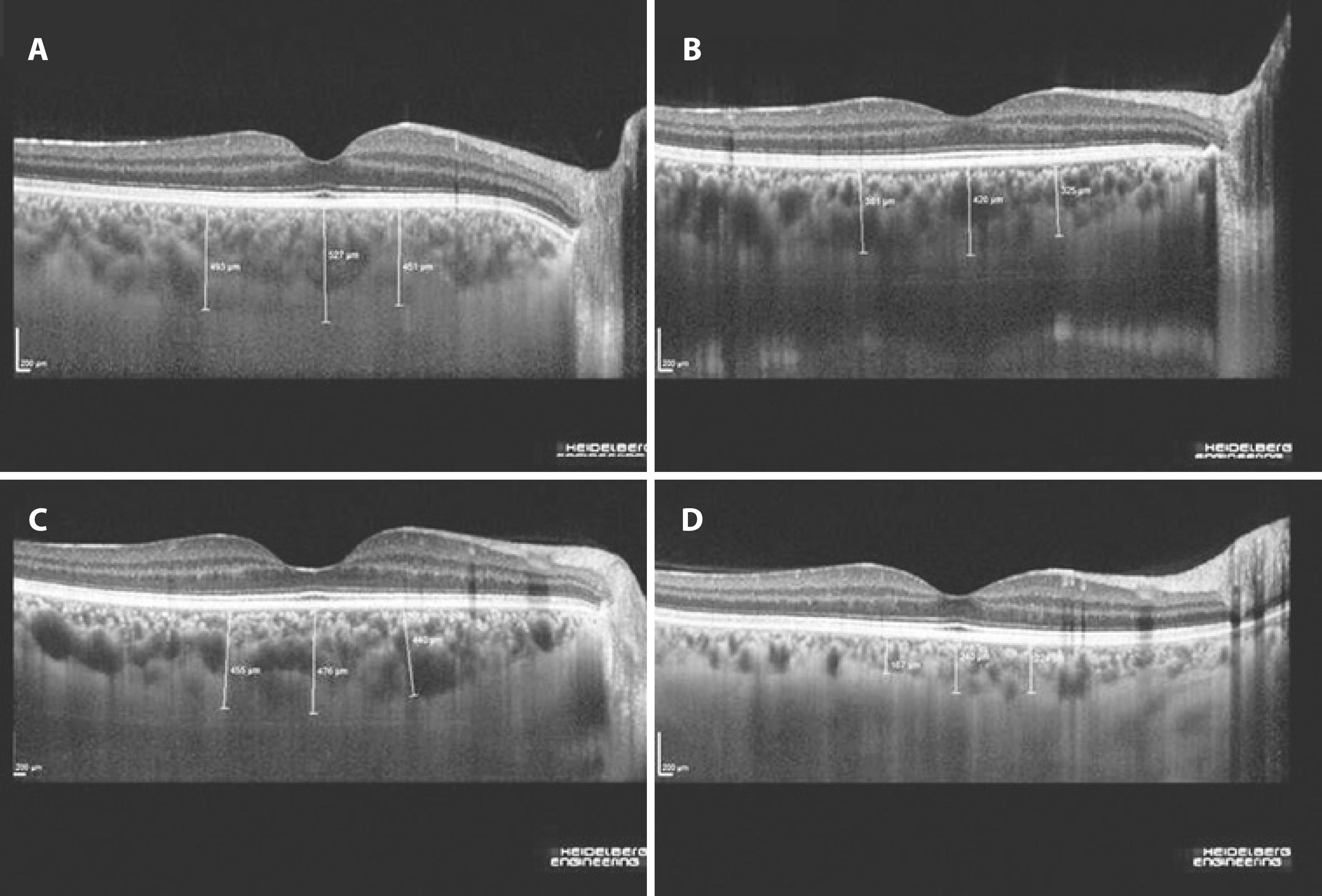
Figure 1 Enhanced depth imaging–spectral domain optical coherence tomography images obtained from patients with inflammatory bowel disease. A) Moderately active ulcerative colitis with an endoscopic activity index of 11. B) Mildly active ulcerative colitis with an endoscopic activity index of 2. C) Moderately active Crohn's disease with a Crohn's disease activity index of 255. D) Crohn's disease on remission with a Crohn's disease activity index of 14.
Statistical analyses
All statistical analyses were performed using SPSS version 15.0 for Windows (Chicago, IL). Normality was assessed using the Kolmogorov-Smirnov test. Accordingly, data were expressed as median (min-max) or mean ± SD. Demographic and clinical characteristics were compared among study groups using analysis of variance for continuous variables and chi-squared analysis for dichotomous variables. Correlation analysis for continuous variables was performed using the Pearson correlation coefficient when indicated. Linear regression analyses were used to compare choroidal thickness among the three groups with adjument for age, gender, and BCVA as these variables have previously been associated with a change in choroidal thickness(19-21). Parameters significantly associated with choroidal thickness based on univariate analysis were incorporated into the regression model. A two-sided P-value of 0.05 or less was considered statistically significant.
RESULTS
Clinical and laboratory indices of study participants are summarize d in table 1 and table 2. Subfoveal (IBD group, 329.4 ± 111.1 µm vs control group, 345.1 ± 85 µm), nasal (IBD group, 297.9 ± 104.8 µm vs control group, 307.1 ± 82.4 µm), and temporal (IBD group, 308.7 ± 116.5 µm vs control group, 329.9 ± 85.2 µm) choroidal thickness measurements were not found to significantly differ between patients with IBD and control subjects (P>0.05). In CD patients, choroidal thickne ss was greater in patients with isolated ileal involvement compared to those with ileocolonic involvement (Table 3, P<0.01), and in patients with ileocolonic involvement compared to those with isolated colonic involvement (Table 3, P<0.05). The only patient with moderately active CD had a significantly greater choroidal thickness compared to patients with mild CD activity or remission (Table 3, P<0.05). Choroidal thickness was similar among UC patients grouped according to disease activity or the site of involvement (Table 3, P>0.05).
Table 3 Mean choroidal thickness measurements in inflammatory bowel disease patients according to disease severity and site of involvement
| Parameter (number of patients) | N3000 | F | T3000 | P-value |
|---|---|---|---|---|
| CD (10) | <0.05 moderate vs mild-remission | |||
| Remission (8) | 257.0 ± 75.9 | 275.0 ± 86.4 | 242.3 ± 69.2 | |
| Mild (1) | 250.0 ± 7.0 | 265.0 ± 14.1 | 240.0 ± 63.6 | |
| Moderate(1) | 445.0 ± 7.0 | 477.5 ± 3.5 | 472.5 ± 74.7 | |
| UC (21) | >0.05 | |||
| Mild (13) | 301.2 ± 106.6 | 334.3 ± 109.4 | 314.1 ± 117.1 | |
| Moderate (7) | 326.6 ± 127.2 | 363.9 ± 130.4 | 356.0 ± 135.3 | |
| Severe (1) | 281.5 ± 40.3 | 375.0 ±l 21.2 | 344.0 ± 48.1 | |
| Crohn's disease (10) | <0.05 among the three groups | |||
| Ileum (6) | 324.7 ± 75.6 | 350.7 ± 82.5 | 316.0 ± 82.9 | |
| Ileum-colon (3) | 221.2 ± 26.8 | 232.0 ± 29.9 | 204.8 ± 49.7 | |
| Colon (1) | 140.0 ± 28.3 | 142.5 ± 10.6 | 140.0 ± 14.1 | |
| Crohn's disease/phenotype (10) | >0.05 | |||
| Perforating (1) | 250.0 ± 7.0 | 265.0 ± 14.1 | 240.0 ± 63.6 | |
| Stricturing (1) | 327.0l± 9.9 | 375.5 ± 29.0 | 289.0 ± 41.0 | |
| Nonperforating/nonstricturing (8) | 271.8 ± 97.9 | 287.8 ± 106.6 | 265.2 ± 104.6 | |
| Ulcerative colitis (21) | >0.05 | |||
| Proctitis (4) | 224.1 ± 61.4 | 280.6 ± 88.1 | 264.9 ± 91.5 | |
| Left-sided colitis (9) | 340.3 ± 121.9 | 367.3 ± 133.9 | 345.7 ± 133.9 | |
| Extensive colitis (6) | 339.7 ± 91.9 | 385.3 ± 80.4 | 366.7 ± 110.8 | |
| Pancolitis (2) | 242.7 ± 95.9 | 264.7 ± 64.6 | 274.0 ± 106.4 |
F= fovea;
N= nasal;
T= temporal.
Numerical values represent mean ± SD.
CD= Crohn's disease;
UC= Ulcerative colitis.
Correlations between choroidal thickness and clinical or laboratory findings were evaluated using Pearson's correlation coefficient. Age, smoking, CD site of involvement (ileal or ileocolonic involvement), CDAI, CD activity, and UC EAI were found to be significantly correlated with choroidal thickness by univariate analysis (Table 4). No correlation was found between choroidal thickness and any other parameter, including erythrocyte sedimentation rate, C-reactive protein level, and platelet number.
Multiple regression analysis revealed that smoking and CD site of involvement (ileal or ileocolonic involvement) were independently associated with increased choroidal thickness at all measurement locations (Table 5).
Table 4 Clinical and laboratory parameters significantly associated with choroidal thickness by univariate analysis and associated Pearson's correlation coefficients
| N3000 | F | T3000 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | P-value | Coefficient | P-value | Coefficient | P-value | ||||
| Age | -0.339 | <0.01 | -0.376 | <0.01 | -0.402 | <0.01 | |||
| Smokinc | -0.297 | <0.05 | -0.337 | <0.01 | -0.371 | <0.01 | |||
| CD* | 0.742 | <0.01 | 0.756 | <0.01 | 0.692 | <0.01 | |||
| CDAI | 0.538 | <0.05 | 0.581 | <0.01 | 0.643 | <0.01 | |||
| CD activity | 0.566 | <0.01 | 0.544 | <0.05 | 0.656 | <0.01 | |||
| UC/EAI | 0.585 | <0.01 | 0.663 | <0.01 | 0.689 | <0.01 | |||
F= fovea;
N= nasal;
T= temporal.
Numerical values represent mean ± SD.
CD= Crohn's disease;
UC= Ulcerative colitis;
CDAI= Crohn's disease activity index;
EAI= endoscopic activity index.
Detailed information regarding individual parameters are presented in tables 1, 2, and 3.
*= site of disease involvement.
Table 5 Multivariate analysis of the effect of potential confounders on choroidal thickness measurements
| N3000 | F | T3000 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | P | B | SE | P | B | SE | P | |||
| Age | -1.76 | 1.62 | 0.30 | -5.0 | 1.6 | 0.01 | -0.70 | 2.00 | 0.74 | ||
| Smoking | 87.70 | 27.20 | <0.01 | 139.5 | 26.7 | <0.01 | 78.60 | 33.90 | 0.04 | ||
| CD involvement* | 102.20 | 16.30 | <0.01 | 88.8 | 16.0 | <0.01 | 93.10 | 20.10 | <0.01 | ||
| CDAI | 0.25 | 0.35 | 0.49 | 0.9 | 0.3 | 0.02 | -0.07 | 0.44 | 0.87 | ||
| CD activity | 6.40 | 44.70 | 0.89 | -66.2 | 43.9 | 0.16 | 75.70 | 55.70 | 0.20 | ||
| UC/EAI | 8.00 | 29.40 | 0.78 | 17.0 | 28.7 | 0.56 | 20.00 | 29.80 | 0.51 | ||
CDAI= Crohn's disease activity index;
EAI= Rachmilewitz endoscopic activity index.
*= site of disease involvement;
B= unstandardized coefficients of the estimated regression model;
SE= standard error of the coefficients;
P= P-value.
DISCUSSION
This study demonstrated that choroidal thickness was similar between patients with IBD and healthy control subjects. No significant correlations were observed between choroidal thickness and clinical, laboratory, and endoscopic parameters of IBD activity. Therefore, choroidal thickness does not appear to have utility as an activity marker in IBD. However, choroidal thickness was found to be greater in CD patients with ileal involvement, a finding that merits further research and discussion.
OCT has been used to evaluate choroid thickness in patients with a number of systemic autoimmune and inflammatory disorders(9-13). Patients with active Vogt-Koyanagi-Harada disease have been shown to have markedly increased choroidal thickness, possibly related to inflammatory infiltration and increased exudation(9). In these patients, increased choroidal thickness and exudative retinal detachment rapidly resolved following treatment with corticosteroids(9). Subfoveal choroidal thickness during the acute phase of uveitis in patients with Behçet's disease has been shown to be greater than during the remission phase, and decreases in choroidal thickness accompanied the amelioration of ocular inflammation in response to infliximab(11). Choroidal thickness was found to be significantly correlated with total, anterior, and posterior inflammatory scores in this study(11). Gungor et al. reported patients with posterior ocular sarcoidosis in the absence of active ocular inflammation had decreased choroidal thickness compared to normal subjects during the quiescent phase of the disease(12), however, the authors were unable to provide a satisfactory explanation for this finding. Kola et al. reported increased choroidal thickness in patients with ankylosing spondylitis compared to healthy controls(13). The authors proposed that chronic systemic inflammation may play an important role in the thickening of the choroid layer in patients with ankylosing spondylitis(13). However, found to no significant correlation between chorodial thickness and systemic inflammatory markers(13).
The eyes are one of the most common sites of extraintestinal involvement in patients with IBD. The majority of patients are diagnosed with IBD prior to the development of ophthalmic complications, however ocular disorders may be the initial manifestion of IBD(22). Uveitis is the most worrisome complication as, if left untreated, it may lead to permanent vision loss. Nongranulomatous, low-grade, recurrent, acute anterior uveitisis is the most common type of IBD-associated uveitis, however posterior uveitis may also occur with chorioretinitis described in 10% of patients in one case series(23). Steroid-responsive choroidal infiltrates and scattered patches of choroidal inflammation with overlying serous retinal detachment have all been previously described in IBD patients(24). Opthalmic complications are associated with active IBD in the majority of cases, but not invariably(25).
To the best of our knowledge, our study is the first to measure choroidal thickness in patients with IBD. No ocular complications were expected in this cohort as none of the patients had previously described any relevant ocular symptoms prior to examination. Although we found significant correlations between choroidal thickness and IBD disease activity (CDAI and UC EAI), these associations were lost after adjustment for covariates. However, ileal involvement remained independently associated with increased choroidal thickness after multivariate analysis.
Ocular complications of IBD are often accompanied by other extraintestinal manifestations, particularly arthritis and erythema nodosum. Similarities between the microvasculature of the synovium and the uvea may play a role in this association as both have a capacity for enhanced antigen presentation, leukocyte migration, and cytokine activity(26). Recent evidence indicates inflammatory responses against a specific colon epithelial protein may lead to cross-reaction against antigens present in the eyes, joints, skin, and biliary tract during the development of extraintestinal manifestations(26). The identification of a similar antigen expressed by ileal epithelial cells with molecular mimicry may explain the increased choroidal thickness observed in patients with ileal involvement in our series. The microvasculature is thought to play a critical role in mediating inflammation. There is growing evidence of widespread inflammatory vasculitis with activation of the clotting cascade leading to microvascular damage and inflammation in the interstitial wall of blood vessels during the development of IBD(27-28). Angiogenesis has emerged as an additional vascular mechanism contributing to chronic inflammation in IBD(29-30). Vasculitic and pro-thrombotic changes and increased angiogenesis may also affect the ocular microvascular bed and mediate the development of ocular complications.
In conclusion, choroidal thickness was not found to have utility as an activity marker in patients with IBD, however ileal involvement in CD was found to be associated with increased choroidal thickness. Further research on the assocation between ileal involvement and choroidal thickness may contribute to our understanding of the pathogenesis of ocular complications in IBD. This study identified smoking as an independent predictor of increased choroidal thickness and should be considered as a potential confounding factor in future studies.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

