INTRODUCTION
Ocular toxoplasmosis is a potentially blinding, necrotizing retinitis and is the most commonly identified cause of posterior uveitis worldwide. Permanent vision loss may occur if lesions affect the macula or optic nerve head. Other complications that can result in visual impairment include persistent vitreous opacities, epiretinal membranes, and cystoid macular edema(1,2).
Visual acuity (VA) is widely used as a means of evaluating central vision based on high-contrast images. However, other fundamental aspects of visual performance, such as contrast sensitivity (CS), color vision (CV), and the visual field (VF), may be impaired in patients with uveitic diseases, despite their VA being essentially normal(3). Some patients with toxoplasmic retinochoroiditis sparing the posterior pole may present with unsatisfactory vision that cannot be detected by high-contrast VA testing. Therefore, measuring other aspects of visual perception can provide additional information regarding the quality of vision among these patients.
The purpose of this study was to evaluate the visual function and architecture of the central and peripapillary retina in patients with inactive toxoplasmic retinochoroiditis outside the macular and peripapillary regions (zones 2 and 3).
METHODS
A cross-sectional study was conducted in 20 eyes of 18 patients with inactive ocular toxoplasmic retinochoroiditis (remission ≥3 months) involving zones 2 and 3 with VA ≥20/25 examined between April 2012 and July 2013 at Altino Ventura Foundation, Recife, Brazil. The study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Ethics Committee. Written informed consent was obtained from all subjects.
Each participant underwent an ophthalmologic evaluation that included measurement of the best-corrected VA (BCVA), slit-lamp evaluation, indirect ophthalmoscopy, intraocular pressure measurement, spectral domain optical coherence tomography (SDOCT) scanning, as well as CS, CV, and VF testing. All examinations for each study participant were performed on the same day.
The topographic location of the retinochoroiditis lesions were recorded using the same classification scheme as for cytomegalovirus retinitis (zone 1: lesions located between the temporal vascular arcades, affecting an area within 3,000 µm of the center of the fovea or 1,500 µm from the edges of the optic disc; zone 2: lesions located outside the boundaries of zone 1 up to the anterior margins of the vortex veins; and zone 3: lesions outside zone 2 up to the ora serrata)(4,5).
Inclusion criteria were as follows: 18 years of age or older, BCVA of 20/25 or better, clear media, spherical refractive error of 0 ± 4.0 diopters (D) and astigmatism of 0 ± 2.5 D, and normal intraocular pressure (<22 mmHg). Patients with diabetes mellitus, uncontrolled systemic hypertension, and glaucoma were excluded from the current study. Participants were also excluded if they had any risk factors for the development of glaucoma or any other eye diseases that could affect the study measurements (such as previous intraocular surgery, previous ocular trauma, and retinal or neurologic abnormalities).
The following data were collected: the length of time since the last episode of active retinochoroiditis, size of the largest retinochoroidal lesion (<1 optic disc area [da] versus ≥1 da), location of the most posterior lesion (zone 2 versus zone 3), and the number of lesions (unifocal versus multifocal). Figure 1 shows a fundus photograph of an eye with toxoplasmic scars ≥1 da in zone 2.
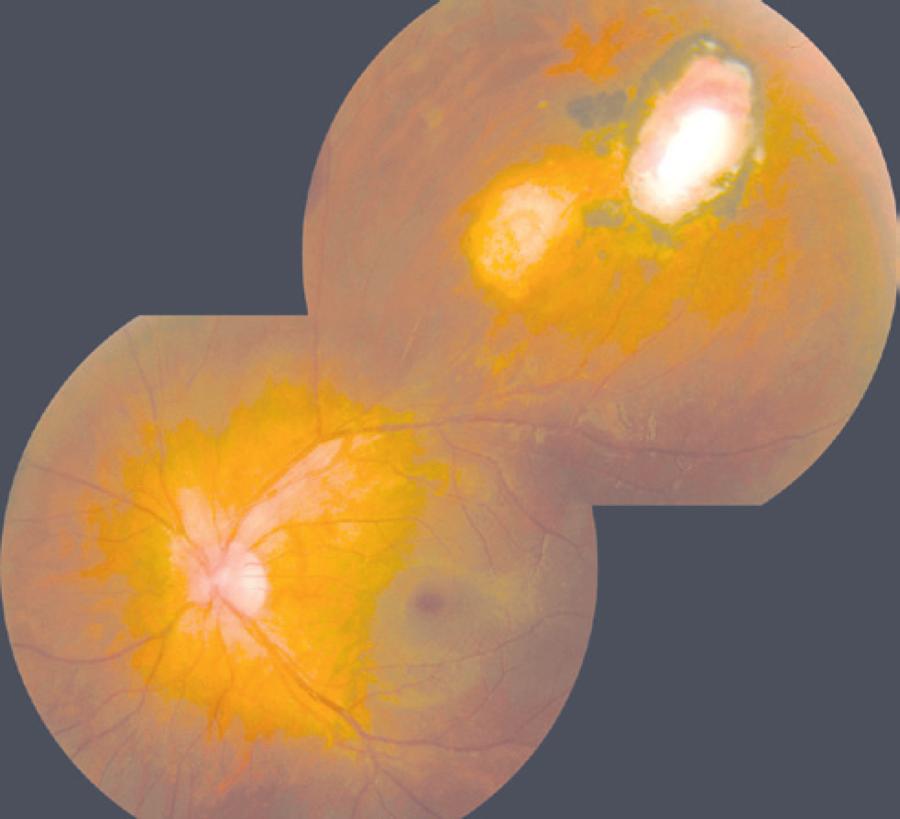
Figure 1 Fundus photograph showing two inactive zone 2 toxoplasmic retinochoroiditis lesions of ≥1 optic disc area.
Optical coherence tomography (OCT) was performed using RTVue SDOCT (Optovue, Inc, Fremont, CA, USA). All patients underwent standard optic nerve head and macular protocols (ONH and EMM5). Only scans with signal strength index ≥45, proper centering, and no evidence of segmentation algorithm failure were included in the analysis.
Contrast sensitivity and CV were tested under standard illumination conditions. Contrast sensitivity was assessed using the Mars Contrast Sensitivity Test (Mars Perceptrix, Chappaqua, NY, USA). The log values of the CS measurements (logCS) were calculated and used in the analysis. A logCS <1.52 was considered abnormal(6). Color vision was determined using the L'Anthony desaturated 15-hue CV test. The color confusion index (C-index) was calculated as described by Vingrys and King-Smith(7). A C-index value of 1.78 or higher was considered abnormal.
Automated static perimetry was performed using the central 10-2 SITA standard program of the Humphrey Field Analyzer (Carl Zeiss Meditec, Dublin, CA, USA). An appropriate lens correction was made for the test distance. Only reliable VFs (false-positive and false-negative results ≤33% and fixation losses ≤20%) were analyzed. Abnormal test results were repeated in order to prevent "first try" effects from interfering with the results. The main outcome measures were mean deviation (MD), pattern standard deviation (PSD), and foveal threshold.
Statistical analyses were performed using the software SPSS 16.0 for Windows (SPSS, Inc., Chicago, IL, USA). The distribution of continuous variables was expressed as mean ± standard deviation and of categorical data as frequencies. Relationships between two categorical variables were assessed using Fisher's exact test. The Mann-Whitney U-test was used to assess the difference in quantitative measurements between groups. Correlations between quantitative variables were analyzed using Spearman's correlation coefficient. A p-value of <0.05 was considered statistically significant.
RESULTS
The mean age of the patients was 27.05 ± 10.62 years, with a mean duration of retinochoroiditis remission of 6.15 ± 5.19 months. Table 1 lists the demographic and clinical characteristics of the study sample.
Table 1 Demographic and medical data of patients with inactive zone 2 and 3 toxoplasmic retinochoroiditis (n=18 patients, 20 eyes)
| Characteristics | Value |
|---|---|
| Gender (18 patients); n (percentage) | |
| Male | 9 (50%) |
| Female | 9 (50%) |
| Age (18 patients; years) | |
| Mean ± SD | 27.4 ± 10.3 |
| Median (range) | 23.5 (18-55) |
| Number of treated retinochoroiditis episodes (20 eyes); n (percentage) | |
| One episode | 18 (90%) |
| Two episodes | 2 (10%) |
| Duration of remission (20 eyes; months) | |
| Mean ± SD | 6.2 ± 5.2 |
| Median (range) | 4.0 (3.0-24.0) |
| Ophthalmic findings (20 eyes) | |
| Location; n (percentage) | |
| Zone 2 | 6 (30%) |
| Zone 3 | 14 (70%) |
| Size; n (percentage) | |
| >1 optic disc area | 11 (55%) |
| ≤1 optic disc area | 9 (45%) |
| Number of lesions; n (percentage) | |
| Unifocal | 13 (65%) |
| Multifocal | 7 (35%) |
SD= standard deviation.
Table 2 shows the macular and peripapillary retinal nerve fiber layer (RNFL) thicknesses as measured by SDOCT. Foveal thickness and mean and quadrant RNFL measurements were within the 95% normal confidence limits of the device's database in all eyes.
The mean logCS was 1.64 ± 0.13, and the mean C-index was 1.41 ± 0.61 (Table 3). The prevalence of abnormal CS and CV was 15.0% (three eyes) and 20.0% (four eyes), respectively.
Table 2 Retinal thickness measured in eyes with inactive zone 2 and 3 toxoplasmic retinochoroiditis (n=20)
| Macula | Peripapillary retinal nerve fiber layer | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Central macula | Parafoveal | Perifoveal | Mean | Temporal | Superior | Nasal | Inferior | ||
| All eyes (n=20) | 255.9 ± 23.6 | 328.8 ± 14.4 | 300.3 ± 15.5 | 116.2 ± 13.8 | 87.5 ± 11.1 | 134.8 ± 20.0 | 89.11 ± 13.5 | 151.4 ± 19.7 | |
| Location | |||||||||
| Zone 2 (n=6) | 257.5 ± 27.4 | 332.5 ± 15.5 | 302.0 ± 18.0 | 117.9 ± 13.5 | 89.8 ± 6.8 | 137.3 ± 20.8 | 94.3 ± 14.3 | 155.1 ± 22.1 | |
| Zone 3 (n=14) | 255.2 ± 22.9 | 327.2 ± 14.1 | 299.6 ± 14.9 | 115.4 ± 14.3 | 86.4 ± 12.7 | 133.6 ± 20.3 | 86.5 ± 12.9 | 149.5 ± 19.1 | |
| p-value* | 0.741 | 0.591 | 0.710 | 0.934 | 0.430 | 0.861 | 0.261 | >0.999 | |
| Size | |||||||||
| ≤1 da (n=9) | 242.1 ± 23.4 | 325.2 ± 16.2 | 302.8 ± 17.7 | 115.0 ± 18.0 | 85.0 ± 10.6 | 130.6 ± 20.7 | 87.3 ± 16.1 | 147.3 ± 24.0 | |
| >1 da (n=11) | 267.2 ± 17.7 | 331.7 ± 12.7 | 298.3 ± 13.9 | 117.1 ± 9.9 | 89.3 ± 11.6 | 137.8 ± 19.8 | 90.5 ± 11.8 | 154.7 ± 16.0 | |
| p-value* | 0.020 | 0.402 | 0.517 | 0.518 | 0.563 | 0.457 | 0.722 | 0.183 | |
| Number | |||||||||
| Unifocal (n=13) | 261.5 ± 18.8 | 331.5 ± 13.2 | 302.1 ± 16.5 | 115.7 ± 14.0 | 84.3 ± 10.7 | 134.7 ± 22.3 | 88.0 ± 14.2 | 150.2 ± 17.2 | |
| Multifocal (n=7) | 245.6 ± 29.5 | 323.7 ± 16.0 | 297.0 ± 13.8 | 117.1 ± 14.4 | 92.9 ± 10.2 | 134.9 ± 16.9 | 90.8 ± 13.2 | 153.3 ± 24.5 | |
| p-value* | 0.219 | 0.266 | 0.691 | 0.905 | 0.128 | >0.999 | 0.684 | 0.821 | |
Values are expressed in micrometers (µm) as mean ± standard deviation;
*= Mann-Whitney U-test;
da= optic disc area.
Table 3 Color vision, contrast sensitivity, and central visual field in eyes with inactive zone 2 and 3 toxoplasmic retinochoroiditis (n=20)
| Color vision | Contrast sensitivity | Central visual field (10-2) | |||||
|---|---|---|---|---|---|---|---|
| (C-index) | (LogCS) | Foveal sensitivity (dB) | MD (dB) | PSD (dB) | |||
| All eyes (n=20) | 1.41 ± 0.61 | 1.64 ± 0.13 | 30.0 ± 9.3 | -4.30 ± 3.9 | 1.9 ± 1.7 | ||
| Location | |||||||
| Zone 2 (n=6) | 1.37 ± 0.39 | 1.62 ± 0.13 | 23.0 ± 12.1 | -5.20 ± 4.7 | 2.1 ± 2.0 | ||
| Zone 3 (n=14) | 1.43 ± 0.69 | 1.64 ± 0.14 | 33.5 ± 3.9 | -3.90 ± 3.6 | 1.8 ± 1.7 | ||
| p-value* | 0.617 | 0.676 | 0.043 | 0.349 | 0.851 | ||
| Size | |||||||
| ≤1 da (n=9) | 1.56 ± 0.85 | 1.69 ± 0.11 | 34.3 ± 26.6 | -3.51 ± 1.0 | 1.5 ± 0.5 | ||
| >1 da (n=11) | 1.30 ± 0.29 | 1.60 ± 0.14 | 26.6 ± 11.2 | -5.00 ± 5.2 | 2.3 ± 2.3 | ||
| p-value* | 0.442 | 0.090 | 0.055 | 0.424 | 0.657 | ||
| Number | |||||||
| Unifocal (n=13) | 1.34 ± 0.42 | 1.65 ± 0.15 | 28.5 ± 11.0 | -4.60 ± 4.8 | 2.1 ± 2.1 | ||
| Multifocal (n=7) | 1.55 ± 0.88 | 1.62 ± 0.11 | 33.0 ± 3.2 | -3.80 ± 0.7 | 1.6 ± 0.4 | ||
| p-value* | >0.999 | 0.445 | 0.638 | 0.223 | 0.061 | ||
*= Mann-Whitney Otest;
da= optic disc area;
MD= mean deviation;
PSD= pattern standard deviation.
The mean perimetric foveal sensitivity was 30.0 ± 9.29 dB, the mean MD was -4.33 ± 3.93 dB, and the mean PSD was 1.91 ± 1.73 dB; the MD and PSD measurements were outside the 95% normal confidence limits of the perimeter's database in 70.0% (14 eyes) and 35.0% (seven eyes) of eyes, respectively.
Foveal sensitivity was lower in eyes with retinochoroiditis in zone 2 than in zone 3 (p=0.041). Eyes with lesions >1 da also had nonsignificantly lower foveal sensitivity (p=0.055). As shown in table 4, there was a positive correlation between the duration of retinochoroiditis remission and MD (r=0.575; p=0.013), and a negative correlation between the duration of retinochoroiditis remission and PSD (r=-0.593; p=0.010). There was also a marginally statistically significant positive correlation between the logCS and the duration of remission (r=0.439; p=0.053).
Table 4 Spearman's correlation coefficients for contrast sensitivity, color vision, and visual field versus duration of remission in eyes with inactive zone 2 and 3 toxoplasmic retinochoroiditis (n=20)
| Color vision | Contrast sensitivity | Central visual field (10-2) | |||||
|---|---|---|---|---|---|---|---|
| (C-index) | (LogCS) | Foveal sensitivity (dB) | MD (dB) | PSD (dB) | |||
| Duration of remission | r=-0.095 | r=0.439 | r=0.098 | r=0.575 | r=0.593 | ||
| p=0.689 | p=0.053 | p=0.700 | p=0.013 | p=0.010 | |||
MD= mean deviation;
PSD= pattern standard deviation.
Spearman's correlation coefficients between visual function parameters and retinal thickness, as measured by SDOCT, are presented in table 5. The correlations between structure and function were mostly non-significant, except for the correlation between parafoveal thickness and MD (r=0.504; p=0.033), and nasal RNFL and the C-index (r=-0.530; p=0.024).
Table 5 Spearman's correlation coefficients for contrast sensitivity, color vision, and visual field versus retinal thickness in eyes with inactive zone 2 and 3 toxoplasmic retinochoroiditis (n=20)
| Color vision | Contrast sensitivity | Central visual field (10-2) | |||||
|---|---|---|---|---|---|---|---|
| (C-index) | (LogCS) | Foveal sensitivity (dB) | MD (dB) | PSD (dB) | |||
| Macula | |||||||
| Central | r=-0.041 | r=-0.178 | r=-0.097 | r=0.444 | r=0.195 | ||
| p=0.862 | p=0.452 | p=0.700 | p=0.065 | p=0.438 | |||
| Parafoveal | r=-0.053 | r=-0.104 | r=0.041 | r=0.504 | r=-0.160 | ||
| p=0.823 | p=0.664 | p=0.873 | p=0.033 | p=0.525 | |||
| Perifoveal | r=-0.142 | r=-0.112 | r=0.175 | r=0.205 | r=-0.086 | ||
| p=0.550 | p=0.637 | p=0.488 | p=0.414 | p=0.734 | |||
| Peripapillary RNFL | |||||||
| Mean | r=-0.138 | r=-0.279 | r=0.044 | r=0.146 | r=0.139 | ||
| p=0.560 | p=0.234 | p=0.864 | p=0.564 | p=0.581 | |||
| Temporal | r=0.020 | r=-0.277 | r=-0.137 | r=-0.012 | r=0.355 | ||
| p=0.934 | p=0.252 | p=0.601 | p=0.963 | p=0.162 | |||
| Superior | r=-0.097 | r=-0.296 | r=-0.023 | r=0.203 | r=-0.105 | ||
| p=0.692 | p=0.218 | p=0.929 | p=0.434 | p=0.687 | |||
| Nasal | r=-0.530 | r=-0.063 | r=0.235 | r=0.244 | r=-0.188 | ||
| p=0.024 | p=0.803 | p=0.381 | p=0.362 | p=0.485 | |||
| Inferior | r=-0.141 | r=-0.217 | r=0.074 | r=-0.126 | r=0.276 | ||
| p=0.576 | p=0.386 | p=0.786 | p=0.641 | p=0.300 | |||
RNFL= retinal nerve fiber layer;
MD=mean deviation;
PSD= pattern standard deviation.
DISCUSSION
Morbidity in uveitic eyes often results from chronic and recurrent episodes of inflammation, and depends on the duration of each episode, the frequency of the attacks, and the anatomic location of the uveitis(8). Ocular toxoplasmosis is a disease that is prevalent worldwide and can lead to legal blindness in at least one eye in approximately 25% of patients(9).
The location of the lesion is the most important cause of VA reduction; scars sparing the central retina, juxtapapillary region, and optic disc rarely interfere with VA(9-11). However, mild-to-severe VF damage is observed even in patients with a VA ≥20/25(10). Toxoplasmic retinochoroidal scars close to the optic disc are associated with absolute VF defects with breakout to the periphery; this is in contrast to lesions that are distant from the disc, which are more likely to produce relative defects(12). In our study, impairment of visual functions (CS, CV, and central VF sensitivity) was documented in patients with zones 2 and 3 retinochoroiditis and good VA.
In the present study, of the 20 eyes analyzed, three had abnormal CS (15.0%) and four had abnormal CV (20.0%). However, we did not find an association between these parameters and the patients' clinical characteristics or retinal thickness measurements. There was a negative correlation between the C-index and nasal RNFL; however, this association could have been due to chance.
Abnormal MD and/or PSD indices (outside the 95% normal confidence limits of the perimeter's database) were found in up to 70% of the eyes. Foveal sensitivity was lower in eyes with retinochoroiditis in zone 2 than in zone 3 (p=0.041), and there was a marginally statistically significant association between lower foveal sensitivity and larger lesions (p=0.055). This indicates that retinochoroiditis closer to the posterior pole, possibly in addition to larger lesions, may induce more retinal damage. In a previous report, lesions greater than 1 da persisted longer and had a higher rate of complications and vision loss than smaller lesions did(13).
A longer duration of retinochoroiditis remission was positively correlated with MD (r=0.575; p=0.013) and negatively correlated with PSD (r=-0.593; p=0.010). This could be associated with the amount of time required by the retina and the optic nerve to recover from the initial insults following adequate control of the inflammatory process, which is also found in uveitic macular edema(8).
Retinal thickness measurements, as assessed by SDOCT, do not justify perimetric changes; the only statistically significant association observed was between MD and parafoveal thickness (r=0.504; p=0.033). Eyes with a history of cystoid macular edema but with normal foveal morphology also present with a reduction in central retinal sensitivity, as measured by microperimetry(14).
The changes observed in the visual function parameters may be associated with optic nerve and macular damage during the inflammatory process, even when these locations are not directly involved with the toxoplasmic lesion. In patients with inactive toxoplasmosis, no correlation was found between the VF defect and the lesion's size and location in 30% of the cases(10). The involvement of the optic nerve in ocular toxoplasmosis has already been described by several authors(1,11,15). In a retrospective study of 926 patients with active ocular toxoplasmosis, 49 (5.0%) patients presented optic disc swelling, and this was most frequently found in association with an active distant focus of retinochoroiditis(11). Macular serous retinal detachment and cystoid changes have also been described in patients with toxoplasmic retinochoroiditis not involving zone 1(16,17). Vitreous inflammation secondary to retinochoroiditis can also induce vitreous haze and disorders of the vitreoretinal interface. Such alterations can resolve without structural damage as detected by commercially available OCT; however, they can be responsible for subtle changes in visual function.
The limitations of our study include the small sample size and the cross-sectional nature of this investigation. Other technologies, including microperimetry and multifocal electroretinography, may also offer valuable and additional information. Another limitation is the subjectivity involved in visual function-testing techniques; we did, however, use standardized protocols (i.e., testing distance, lighting conditions, VF reliability parameters) to minimize this problem. Ocular toxoplasmosis is a relapsing disease; however, most of our patients only presented with one uveitic episode (although 35% presented with multifocal lesions). Cumulative damage associated with multiple relapses could have allowed for the detection of more severe changes.
CONCLUSION
Eyes with inactive zone 2 and 3 toxoplasmic retinochoroiditis and with VA ≥20/25 can present with abnormal color, contrast, and macular perimetric sensitivity despite normal macular and peripapillary retinal architecture. Zone 2 retinochoroiditis was associated with lower foveal threshold sensitivity. A longer duration of remission for ocular toxoplasmosis was associated with improvements in perimetric parameters (MD and PSD).




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

