INTRODUCTION
A. Francheschetti and J. Babel published the first case of birdshot retinochoroiditis, called "La chorio-rétinite en taches de bougie, manifestation de la maladie de Besnier-Boeck"( 1 ), in 1949. This new syndrome and entity in intraocular inflammation was given the name birdshot retinochoroidopathy (BSRC) in 1980. The unique features of this disease are minimal anterior segment inflammation, and diffuse posterior choroidopathy associated with vitritis and retinal vasculitis( 2 , 3 ). Gass named birdshot lesions vitiliginous chorioretinitis because of the appearance and evolution of the patches of choroidal depigmentation and the hypopigmented skin lesions in patients with vitiligo( 4 ). Foster and associates evaluated the records of 1,237 patients with uveitis referred to a large tertiary eye center in the United States from 1982 to 1992. They reported that nineteen of 240 patients with posterior uveitis had BSRC. In this series, BSRC was the fifth most common subtype of uveitis causing posterior uveitis with a prevalence of 7.9%( 5 ).
In 2006, an international consensus group enunciated criteria for the diagnosis of BSRC. Data were obtained from investigators who attended this conference. Sensitivity and specificity of the criteria were evaluated by reviewing 82 previously diagnosed cases and evaluation of 80 consecutive patients with other forms of uveitis. The sensitivity of the consensus was not 100%, but provided a homogeneous group of patients with BSRC for research purposes( 6 ).
Numerous therapies and different groups of drugs have been used to treat BSRC. The objective of this review is to increase attention to this rare and vision threatening eye disease, specifically focusing on the matters of the new discoveries, immune mediators, current treatments, new therapies and techniques to achieve and monitor remission.
PATHOGENESIS
Birdshot retinochoroidopathy inflammation is at the level of the retina and the choroid, induced by an unknown trigger. The target auto-antigens are at the level of the pigmented layer of the retina, hence the disease is considered a retinochoroidopathy rather than a chorioretinopathy( 7 ).
There is a strong association between the HLA-A29 gene and BSRC. HLA-A29 gene positivity is a supportive finding but not a required criterion for diagnosis( 6 ). The prevalence of HLA-A29 is only 7% in the general population, but 80-95% among BSRC patients( 8 - 10 ). The HLA-A29 gene can be divided into two subtypes: HLA-A29.1 and HLA-A29.2. Although both subtypes are documented in BSRC, HLA-A29.2 has a higher prevalence( 10 ). HLA-A29.2 gene products are thought to interfere with T cell receptor mediated T cell NK cell interactions, leading to activation of T cell subsets against intraocular self-antigens( 11 , 12 ).
Immune mediators in intraocular fluids and serum samples during different phases of BSRC have been studied to better understand the role of T cells in BSRC( 13 - 15 ). Twenty-three immune mediators were analyzed in intraocular fluid and peripheral blood samples of 16 BSRC patients. Results were reported indicating that BSRC patients have elevated intraocular levels of proinflammatory and T cell-associated cytokines. The study also suggested that BSRC is a novel disease restricted to the eye and associated with elevated levels of IL-17( 14 ).
Kuiper et al. analyzed T cell cytokine production by lymphocytes in the peripheral blood of BSRC patients. They reported that the T lymphocyte cytokine production was followed by the proliferation of IL-17-producing CD4+ T lymphocytes( 15 ). Yang and Foster analyzed the serum levels of interleukins (IL) and transforming growth factor (TGF) in BSRC patients. The results revealed that patients with active HLA-A29-associated BSRC without previous systemic therapy had elevated serum levels of IL-21, IL-23, and TGF-β1, illustrating the importance of systemic therapy and further investigations to design new treatments( 13 ).
Regulatory T lymphocyte (Tregs) are involved in the development of systemic autoimmune disorders, and influence autoreactive T cells, as seen in Behcet’s disease and Vogt-Koyanagi-Harada syndrome( 16 , 17 ). Recently, Foster et al. evaluated the percentage of Tregs in the peripheral blood of patients with active BSRC. Leukocytes CD4+, CD25+, and Foxp3+ Tregs are indispensable cellular constituents of the normal immune system. For the first time it was reported that CD4+, CD25+, Foxp3+ and Tregs may be involved in the regulation of ocular self-tolerance in BSCR patients. These findings present opportunities for developing new therapeutic strategies for ocular inflammatory diseases( 18 ).
CLINICAL PRESENTATION AND DIAGNOSIS
Birdshot retinochoroidopathy diagnosis is ultimately based on clinical examination (Table 1). The clinical criteria for diagnosis involve the following: (1) Required findings: bilateral evidence of low-grade anterior chamber inflammation, low-grade vitreous inflammation, and three or more peripapillary hypopigmented lesions at the level of the choroid (characteristic birdshot lesions) (Figure 1) inferior or nasal to the optic disk; (2) Supportive findings: HLA-A29 positivity, retinal vasculitis, and cystoid macular edema; (3) Exclusion criteria: infectious or neoplastic diseases that present multifocal choroidal lesions. Herbort and others have reported granulomatous keratic precipitates (KPs) in BSRC patients. Thus, KPs are not part of the exclusion criteria( 19 ).
Table 1 The clinical criteria for Birdshot retinochoroidopathy diagnosis
| Required findings: |
| Bilateral evidence of low-grade anterior chamber inflammation |
| Low-grade vitreous inflammation |
| Three or more peripapillary hypopigmented lesions at the level of the choroid inferior or nasal to the optic disk (characteristic birdshot lesions). |
| Supportive findings: |
| HLA-A29 positivity |
| Retinal vasculitis |
| Cystoid macular edema |
| Exclusion criteria: |
| Infectious or neoplastic diseases presenting with multifocal choroidal lesions. |
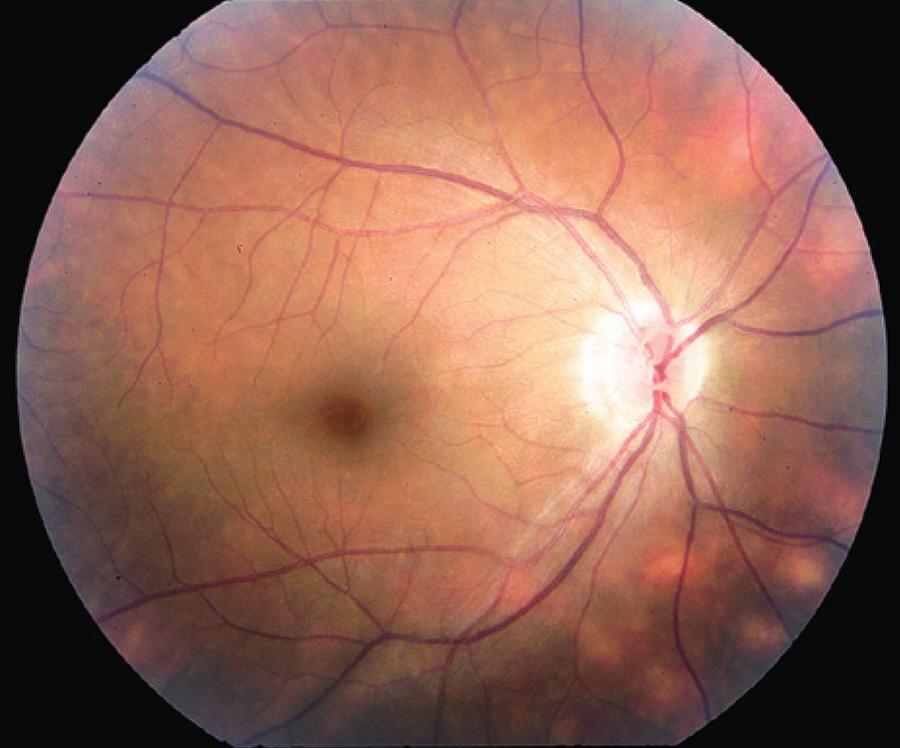
Figure 1 Typical "birdshot lesions" (cream-colored choroidal lesions with indistinct borders) in an individual with birdshot retinochoroidopathy.
BSRC is predominantly an ocular disease affecting otherwise healthy patients, without a consistent association with a systemic disease. Typically, the onset of visual symptoms involves one eye, but over time the other eye is also affected. At initial presentation, patients commonly complain of blurry vision and floaters but usually have good visual acuity( 20 ). Color vision changes, photophobia, flashes, glare, and nyctalopia have also been reported( 2 - 4 , 20 - 24 ).
DIFFERENTIAL DIAGNOSIS
In some cases of BSRC, classic choroidal lesions may not be present, mimicking other posterior uveitis entities. Differential diagnosis includes infectious and non-infectious systemic diseases that cause panuveitis and light colored fundus lesions (white dot syndromes).
Syphilis and tuberculosis can cause vitritis and chorioditis with light colored fundus lesions. However, these lesions have typical features with varying degrees of retinal pigment epithelium (RPE) hyperplasia distinguishable from BSRC lesions( 25 , 26 ). Moreover, history, systemic presentation, serologies, skin test and chest X-ray are utilized to differentiate these diseases. Ocular histoplasmosis syndrome also has a similar clinical presentation. However, it has distinct clinical characteristics of peripheral histo spots, peripheral pigmentary changes, and maculopathy without vitritis( 26 ). Diffuse unilateral subacute neuroretinitis (DUSN) presents with unilateral gray-white lesions and vitritis( 27 ).
Ocular sarcoidosis manifests with vitritis, vasculitis and choroidal granuloma, although typical anterior chamber granulomatous inflammatory findings might be absent in some cases( 28 ). Ocular sarcoidosis must be ruled out during BSRC work-up. Vogt-Kyonagi-Harada (VKH) disease also manifests with choroidal lesions and bilateral intraocular inflammation. Extraocular involvement along with serous retina detachment and choroidal thickening are characteristic findings in this disease( 29 ). Sympathetic ophthalmia is another chronic ocular inflammatory disease that presents with choroidal creamy colored foci. However, a history of ocular trauma or surgery will differentiate it from BSRC( 30 ).
Multifocal choroiditis and panuveitis (MCP) syndrome with punctate inner choroidopathy (PIC), multiple evanescent white dot syndrome (MEWDS), and acute multifocal placoid pigment epitheliopathy (APMPPE) are part of the white dot syndromes family, and can be distinguished from BSRC based on the shape, size, color, and location of the lesions( 31 , 32 ). HLA-A29 antigen assessment is recommended for cases of vitritis, vasculitis, and papillitis with or without the typical BSRC lesions, due to the possibility of delayed development of these lesions in some patients( 33 ). Masquerade syndrome, particularly intraocular large-cell lymphoma, can manifest with BSRC lesions. However, it has a distinguishable clinical context with distinctive size and location of the lesions. Serology, lumbar puncture, brain MRI, and diagnostic vitrectomy are required to confirm lymphoma diagnosis( 34 ).
TREATMENT
Long-term monotherapy with corticosteroids is inadequate to induce durable remission in BSRC( 35 - 37 ). The efficacy of regional steroid injections is transient and limited to treating acute inflammatory exacerbations and macular edema. Early administration of immunomodulatory agents is recommended to prevent disease progression and vision threatening complications( 22 ). A variety of immunosuppressive agents, including cyclosporine( 38 ), methotrexate( 39 ), azathioprine( 40 ), derivatives of mycophenolic acid( 41 ), intravenous immunoglobulin( 42 ), anti-tumor necrosis factor alpha (Anti-TNF α) inhibitors( 43 ) and daclizumab( 44 ) have been administered as monotherapy or as combination therapy in the treatment of BSRC.
Despite successful reports of high dose cyclosporine mono-therapy (10 mg/kg/day) in BSRC disease control, it is associated with nephrotoxicity and hypertensive side effects( 45 ). To reduce the adverse side effects, low dose cyclosporine (2.5-5 mg/kg daily) alone or combined with azathioprine or mycophenolate mofetil is recommended. A retrospective study by Vitale et al. reviewed the efficacy of cyclosporine (3 mg/kg) and mycophenolate mofetil (1 gram twice daily) combination therapy in BSRC. Only two patients developed elevated blood pressure, which resolved with antihypertensive treatment adjustment or cyclosporine dosage reduction( 38 ). Overall, sixty seven percent of patients achieved remission during the first year of treatment, and this combination therapy was concluded to be an efficacious option for treating BSRC.
Anti-TNF α inhibitors (e.g. infliximab) have been increasingly utilized in treating non-infectious uveitis( 46 ). However, the effect of infliximab in BSRC has been reported only in small case series( 47 ). Artornsombudh et al. reported infliximab to be effective in 88.9% of 22 patients with BSRC refractory to traditional immunosuppressive therapy (IMT) after 1 year of treatment. This result reflect the adequacy of infliximab among refractory cases of BSRC( 48 ).
In a prospective study, Cassoux et al. evaluated the efficacy of intravenous polyclonal immunoglobulin (IV-Ig) in 18 cases of BSRC. In 61% of the patients they observed improvement of inflammation as determined by fluorescein angiography, and cystoid macular edema resolution. IV-Ig was well tolerated with minimal side effects, thus indicating that IV-Ig is a safe and efficacious alternative treatment for BSRC( 42 ).
For BSRC patients who are unable to tolerate systemic therapy, a sustained-release fluocinolone acetonide intravitreal implant (Retisert®) is a possible therapeutic alternative. In a retrospective study, Bajwa et al. confirmed the effectiveness of the Retisert® implant in treating active vasculitis, macular edema and its ability to reduce IMT during the course of three years. However, a majority of the patients developed elevated intraocular pressure and cataracts( 49 ).
New treatment modalities for BSRC have been proposed targeting the recently discovered pathogenesis. T-lymphocytes have been found to play a crucial role in BSRC, especially Th17 and IL-17 produced by T- lymphocytes. Also, elevated levels of IL-23 and TGFβ-1 have been reported( 50 ). The following medications are the new upcoming therapeutic modalities for recalcitrant BSRC:
Daclizumab is a humanized immunoglobulin G monoclonal antibody directed against the CD25 subunit of the IL-2 receptor complex of T-cells. It was shown to be effective in suppressing intraocular inflammation( 50 ), specifically in reducing vitreous inflammation and improving visual acuity among BSRC patients( 44 ).
Ustekinumab is a humanized monoclonal antibody directed against IL-12 and IL-23, targeting the Th17 and Th1 pathways. Currently there is an ongoing pilot study of Ustekinumab in active sight-threatening uveitis, expected to be completed in 2015( 51 ).
Tocilizumab is a humanized monoclonal antibody directed against IL-6 receptor. The available administration methods are subcutaneous injections and intravenous infusions. It is reported to be effective against juvenile idiopathic arthritis associated uveitis( 52 ).
Fingolimod is an immunomodulatory agent. It reduces antigen presentation by blocking lymph node efflux through the sphingosine-1-phosphate receptor. Zarbin et al. observed that when fingolimod was used to treat multiple sclerosis, it induced macular edema in patients with pre-existing uveitis( 53 ).
Natalizumab is a humanized monoclonal antibody directed against α4-integrin that reduces inflammatory cell migration. It is currently approved to treat Crohn’s disease( 54 )and multiple sclerosis( 55 ). Clinical trials evaluating its efficacy in non-infectious uveitis have not yet been performed.
Gevokizumab is a monoclonal antibody directed against IL-1β. It is currently in phase 3 of the clinical trials EYEGUARD™-A( 56 )and EYEGUARD™-C( 57 ).
Anakinra is an IL-1β antagonist used in chronic infantile neurological cutaneous articular syndrome associated posterior uveitis( 58 ). Clinical trials evaluating its application among non-infectious uveitis have not been developed.
Interferon α-2a therapy was reported to be effective in Behcet’s disease associated uveitis( 59 ).
Currently, monoclonal antibodies against CD4, CD8, IL-23, Th 17, and ERAP2 gene (endoplasmic reticulum aminopeptidase 2) are under investigation.
MONITORING CONTROLLED DISEASE TO REACH REMISSION
The layers of the retina and the choroid in BSRC are described to be independently affected( 60 - 64 ); however, progressive deterioration also occurs as the disease advances. The outer retina and choroid are the primary anatomic structures damaged( 65 , 66 ). Numerous methods are used to monitor the progression and responses to treatment of the disease. Pathologic manifestations are identified with clinical observations( 63 ) and ocular imaging including fundus fluorescein angiography (FA), fundus autofluorescence (FAF), indocyanine green angiography (ICG), and ocular coherence tomography (OCT). The functional status of the disease is evaluated with an electroretinogram (ERG)( 67 , 68 ). The most practical and frequent ophthalmic multi-modality imaging techniques are FA, ICG, OCT, FAF, ERG and the Swedish interactive threshold algorithm with short-wavelength automated perimetry testing (SITA-SWAP) visual field test( 69 , 70 ).
MULTIMODALITY IMAGING TECHNIQUES
Fluorescein angiography is a useful method for assessing disease activity, specifically BSRC complications such as papillitis, retinal vasculitis, and cystoid macular edema (CME). However, it has relatively low specificity in characterizing birdshot lesions (Figure 2). The reported incidence of CME in BSRC patients is as high as 34-42%( 71 , 72 ). Retinal vasculitis and CME are supportive rather than diagnostic findings of BSRC, thus an FA is performed on all patients, regardless of clinical evidence of retinal vasculitis or CME. FA is an essential player for monitoring disease activity during the follow up period, recommended every 6 months at a minimum. Additionally, FA also offers information to detect rare vision threatening complications such as choroidal neovascularization (CNV)( 63 , 69 ).
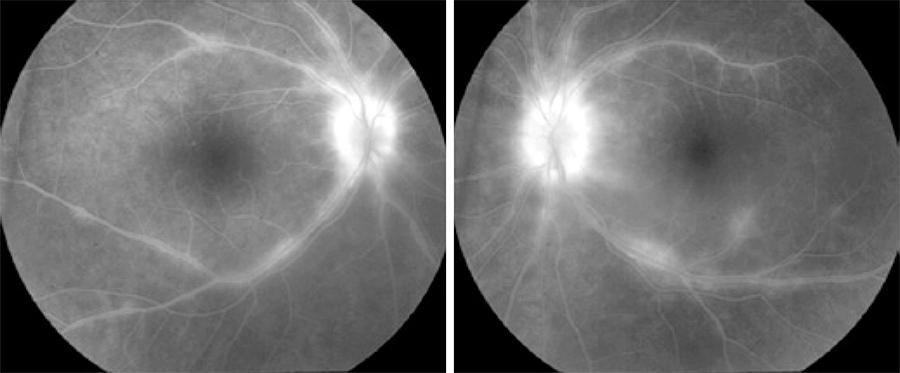
Figure 2 Fluorescein angiogram revealing bilateral phebitis and papillitis in a case of birdshot retinochoroidopathy.
Indocyanine green angiography is more sensitive than FA for detecting BSRC lesions. Areas of choroidal vascular nonperfusion and inflammation are appreciable with an ICG as hypolucent round or oval lesions( 58 , 69 , 70 )(Figure 3). In late-stage disease, when lesions are more atrophic, they appear more isofluorescent, better visible, and more numerous on ICG than on ophthalmoscopic examination. In late phase, the lesions either remain hypofluorescent or become isofluorescent. During active disease, additional features of indistinct choroidal vessels and late diffuse hyperfluorescence are observed( 20 ). It is recommended to obtain ICG on the first visit and to repeated it as needed.
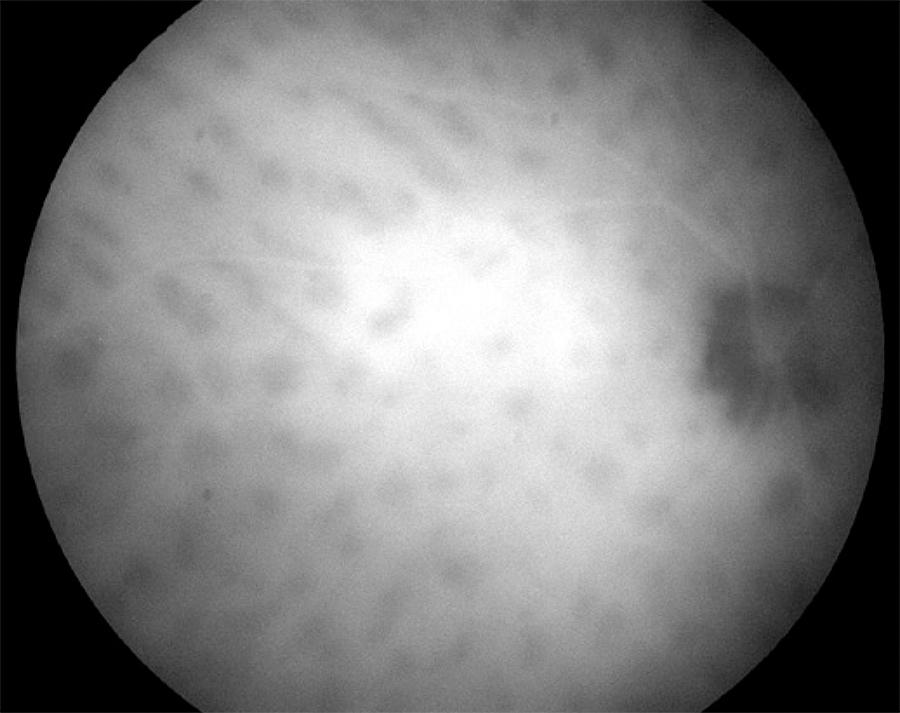
Figure 3 Indocyanine green angiogram detecting hypolucent round lesions corresponding to areas of choroidal vascular nonperfusion.
Ocular coherence tomography is an appropriate measure not only to diagnose macular edema, but also to depict macular thinning or atrophy correlating with abnormal visual function( 71 , 73 ). As the disease progresses, thinning of the choroidal vascular layer and atrophy of the outer retina will ensue. In addition, restoration of retinal physical structure is not an uncommon finding when the correct immunosuppressants are used( 64 ). Unlike other investigations, such as FA, ERG, and SITA-SWAP VF testing, it is not necessary to obtain OCT every 6 months. Rather, macular OCT is recommended when CME or macular atrophy are suspected (Figure 4).
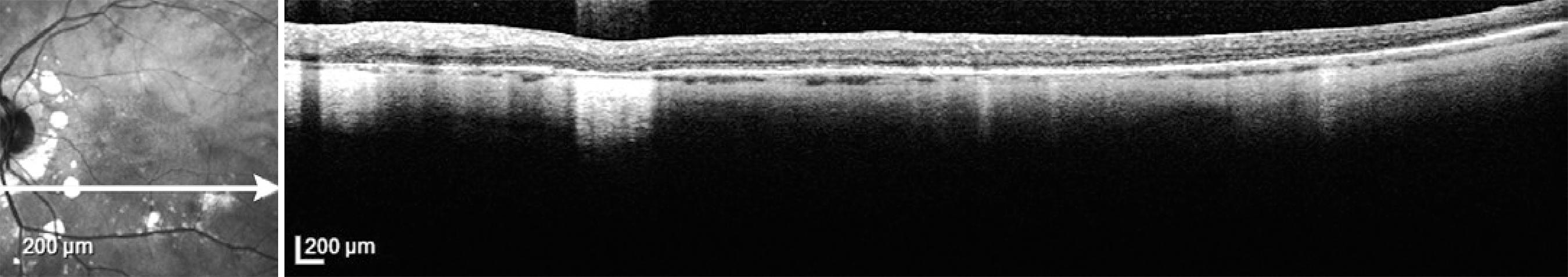
Figure 4 Optical coherence tomography of the left eye showing localized areas of retinal pigment epithelium atrophy (BSRC lesions) and thinning of the choroid in a patient with birdshot retinochoroidopathy.
Fundus autofluorescence is superior to clinical examination because of its ability to display hypofluorescent spots (Figure 5) along with the advantage of correlating its findings with OCT and visual field changes( 62 , 74 ). However, the capability to monitor disease progression requires further investigation, given the limited evidence of its prognostic value. FAF is not required routinely to monitor BSRC patients.
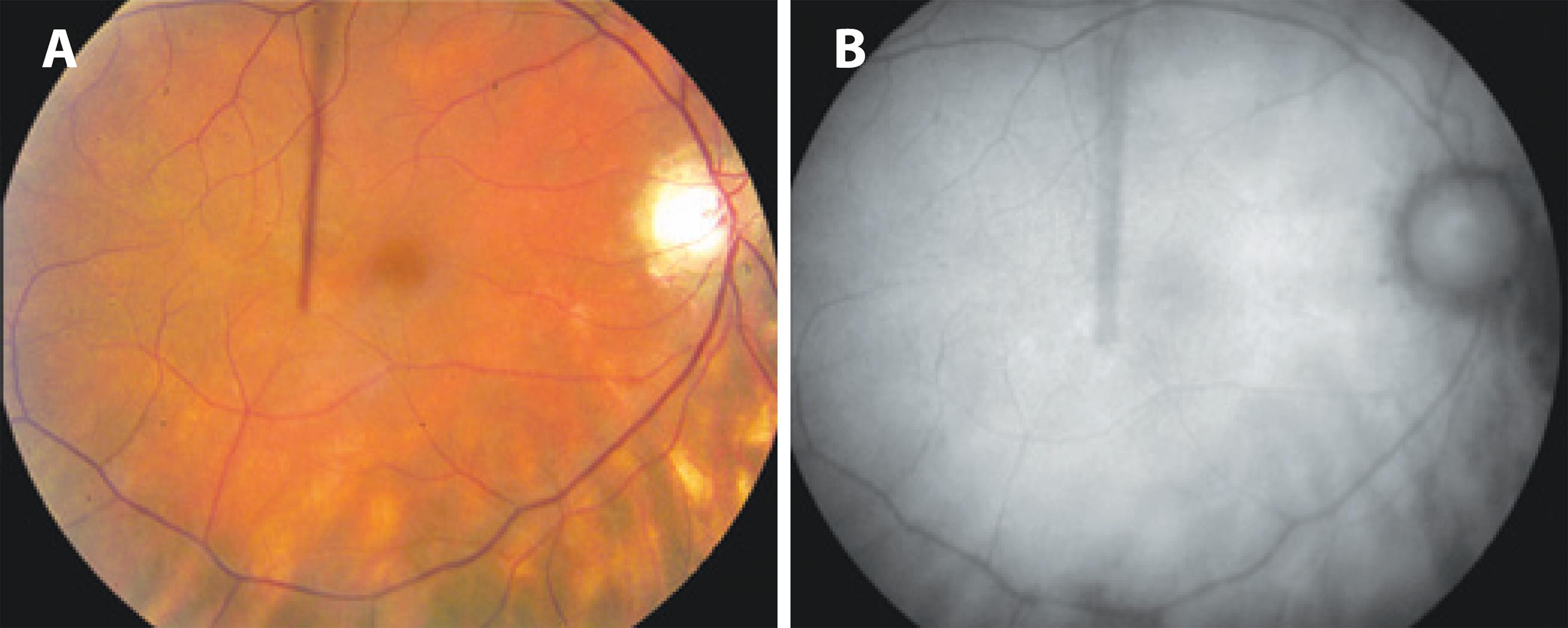
Figure 5 A) Fundus color photograph shows poorly pigmented fundus with BSCR lesions nasal and inferior to the optic nerve. B) Fundus autofluorescence shows scattered areas of retinal pigment epithelium atrophy as hypoautofluorescent spots corresponding to the BSCR lesions visible in the color photograph.
Electroretinography is not only one of the most sensitive and imperative tools in the diagnosis of BSRC and monitoring disease activity by detecting subtle functional retinal changes( 22 , 75 ), but it is also an excellent method to determine the response to treatment( 70 ). ERG findings are superior to visual field findings for predicting disease response. The changes detected with a 30 Hz cone flicker implicit time is a profoundly useful modality to recognize disease activity( 76 ). It is the most sensitive and consistently affected ERG parameter( 70 ). Restoration of flickering implicit time is a common finding following disease improvement( 76 , 77 ). Although ERG is time-intensive and requires an experienced operator, biannual monitoring with a 30 Hz flickering ERG is recommended.
Visual field test: despite the lack of consistency in visual field assessment between different study groups, extensive visual field deterioration coexists with relatively well-preserved central visual acuity( 78 ). Visual field assessment is a useful modality for monitoring disease activity and monitoring response to treatment in BSRC. Specifically, SITA-SWAP is the most sensitive type of visual field testing for early detection of visual field changes. A vast spectrum of scotoma patterns has been observed. Changes in the characteristics of a scotoma indicate active disease (Figure 6).
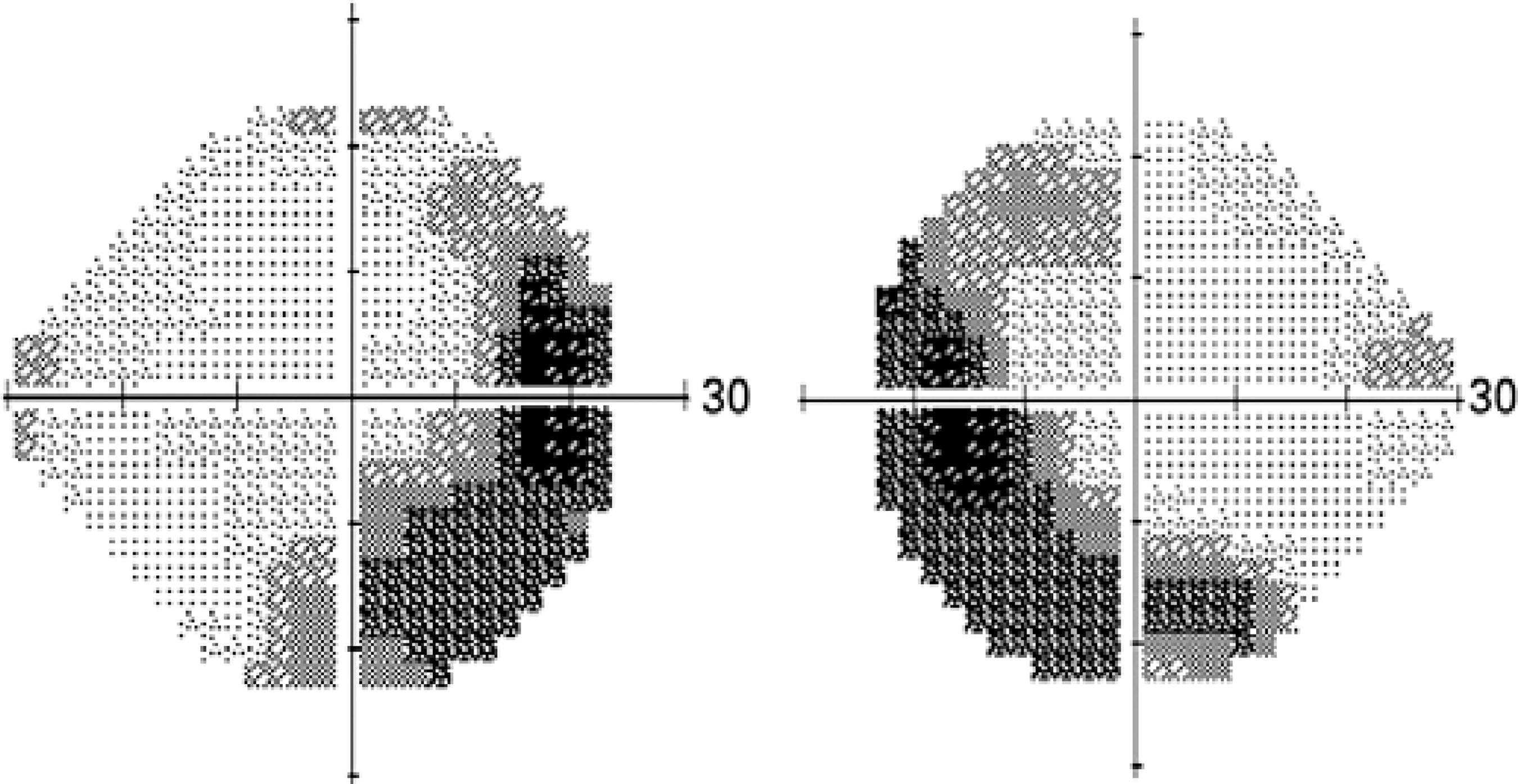
Figure 6 Visual field test (SITA-SWAP) showing peripheral visual field changes in active birdshot disease.
Multiple components are involved in achieving durable disease remission. The most important component is the employment of IMT until 2 consecutive years of steroid-free remission have been reached before considering to end IMT ( 70 , 79 ). All complementary tests are recommended when active stage of the disease is suspected, or repeated routinely while in remission. In our clinic, approximately 200 cases of BSRC are monitored routinely with FA, ICG, ERG, and SITA-SWAP every 6 months. Ocular coherence tomography may be considered when CME or macular atrophy is suspected. Therefore, diagnostic tests are repeated every 6 months or more often when relapse or recurrence of the disease is suspected.
CONCLUSION
Birdshot retinochoroidopathy is an autoimmune inflammatory disease restricted to the eye, with a strong association with the HLA-A29 gene. It is also associated with elevated systemic levels of IL-17, IL-21, IL-23, and TGF-β1, highlighting the systemic involvement of the disease and hence the importance of systemic therapy. Administering traditional immunosuppressive agents such as cyclosporine and mycophenolate mofetil to accomplish 2-year steroid-free remission, and then slowly tapering to completion, has shown encouraging results for adequate treatment of BSRC. Biologics are considered for uveitis refractory to traditional IMT. Appropriate treatment and rigorous monitoring for this sight threatening disease can achieve remission and, in many cases, a cure.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

