INTRODUCTION
Many studies have contributed to the development of foldable IOLs of different materials and designs, and have been performed to help cataract surgeons to select an IOL that could provide the best quality of vision for pseudophakic patients(1). Some of those studies have shown that the human cornea has a positive value of spherical aberration (SA), which is stable throughout the entire life(2).
Implantation of a spherical IOL could theoretically increase the positive SA of the eye and subsequently degrade the quality of vision(3-5). The aspheric IOLs were developed as an option to generate less higher-order aberration (HOAs) after cataract surgery, providing patients a better contrast sensitivity (CS) and, therefore, enhancing patients’ quality of life(6-10).
Frequency-doubling technology (FDT) testing for the detection of glaucomatous visual field (VF) loss consists of determining CS measures for detecting the frequency-doubled stimulus(11). Maddess et al.(12) proposed that a CS test based on the frequency-doubling illusion should be effective in the detection of early glaucomatous VF loss. However, previous studies have yielded divergent views regarding the diagnostic performance of FDT perimetry to standard automatic white-on-white perimetry (SAP) for the detection of glaucoma(13-15).
Because previous studies have demonstrated an improvement in CS in eyes implanted with aspheric IOLs(6-10), is important to investigate its influence on the results of FDT. As the number of aspheric IOLs implanted all over the world increases and the FDT is progressively incorporated in clinical practice, understanding the effect of aspheric IOLs on this mode of perimetry is of considerable clinical importance. A possible improvement in the perimetry values with such lenses may mask the diagnosis of glaucoma or lead to other diagnostic confusions.
The aim of the present prospective randomized clinical study was to investigate whether implantation of an aspheric IOL results in reduced ocular aberrations and improved CS (measured using the VCTS 6000) after cataract surgery and, therefore, changes in FDT values.
METHODS
The present prospective, randomized double-blinded study included 25 patients with bilateral cataracts. Patients randomly received an aspheric IOL Akreos AO in one eye (25 eyes; Bausch & Lomb, Inc), and a spherical IOL Akreos Fit (25 eyes; Bausch & Lomb, Inc.) in the other eye. The present study was conducted in adherence with the tenets of the Declaration of Helsinki, and approval of the study was obtained from the institutional review board of the Clinical Hospital, São Paulo, Brazil. All patients received a detailed explanation of the study, including the necessity for an examination, after which they provided written informed consent.
Patients without any kind of glaucoma observed during the ophthalmologic examination were eligible for inclusion in the study. The optic discs were evaluated and the retinal nerve fiber layers were examined using Cirrus optical coherence tomography (OCT; Carl Zeiss Meditec) and fundus photography. Exclusion criteria were any other ocular disease, such as corneal opacities or irregularity, amblyopia, retinal abnormalities, surgical complications, posterior capsule opacification or incomplete follow-up. Ocular evaluations (visual acuity, intraocular pressure measurements, gonioscopy, central corneal thickness and fundus examination) were performed in each study visit as a basic visual care parameter to avoid bias of low visual acuity on FDT perimetry and to ensure that glaucomatous patients were not included.
Patients were examined before surgery and at one, seven and 30 days, and three, six and 12 months after surgery. At 12 months, best-corrected visual acuity (BCVA), distance uncorrected visual acuity (UCVA), CS, wavefront analysis and FDT values were measured.
Visual acuity was measured using the Early Treatment of Diabetic Retinopathy Study charts under photopic conditions (target luminance of 85 cd/m2). The values were converted to the logarithm of the minimal angle resolution scale (logMAR).
CS was measured using the VCTS 6000 (Vistech Consultants Inc, Dayton, Ohio, USA) with best-spectacle correction under photopic (85 cd/m2) and mesopic (3 cd/m2) conditions. Light conditions were controlled using a luxometer (Gossen-Starlite, Nürnberg, Germany). The log base 10 CS values were used to construct a graphic for each spatial frequency tested and then presented in the original test scale.
Wavefront analysis was performed using the OPD-Scan aberrometer (Nidek Co, Gamagori, Japan), which uses dynamic retinoscopy technology to obtain wavefront data. Measurements were repeated at least three times to obtain a well-focused and aligned image of the eye, and analyzed for 5 mm and 6 mm pupils. All aberrations were measured up to the sixth Zernike order.
Pupils were dilated using two drops of cyclopentolate 1% given 15 min apart, and the size measurements (in millimeters) were obtained 45 min after the last cyclopentolate drop was administered (under scotopic [1.5 cd/m2], mesopic [3 cd/m2], and photopic [85 cd/m2] conditions). Pupil diameter was measured using the Colvard pupillometer (Oasis Medical, Glendora, California, USA).
FDT perimetry (Carl Zeiss Meditec, Dublin, CA, USA) was performed using best-spectacle correction in a darkened room (target luminance of 3 cd/m2). Patients were assessed using the N-30 full-threshold program. The strategy tested four locations in each quadrant (10º × 10º squares), one central location (10º of the arc of a central circle) totaling 17 locations tested in the central 20º and two in the lateral 30º VF. In each area, the FDT device presents frequency-doubling stimuli with low spatial frequency, co-sinusoidal grating (0.25 cycles/ degree) and high temporal frequency (25 Hz counter-phase flicker) on a square background.
The N-30 full-threshold protocol determines the minimum contrast necessary to detect the stimulus for each of the 19 target locations in the stimulus display. This is accomplished by means of a modified binary search (MOBS) type of staircase. If the stimulus is detected, the contrast is decreased in the following presentation; if the stimulus is not detected, the contrast is increased. Thus, the test is continued until the stimulus with the lowest contrast is detected(16).
Parameters on FDT included in the analysis were mean deviation (MD) and pattern standard deviation (PSD). Only reliable fields were accepted in the study (<33% false-positive or false-negative responses and <20% fixation losses).
A screening test (C-20-5) was first performed to minimize the learning curve. An interval of at least 5 min was allowed between the screening test and the full-threshold test in order to improve reliability, as suggested by previous studies(17). These tests were not included in the analysis.
In addition, the VF was also divided into four areas (Figure 1) to evaluate the variation of the values in different areas: the central area (A1); the 10º field area (A2); the 20º field area (A3) and the 30º lateral field area (A4). The median thresholds for each of the four areas were calculated; each of these medians are referred to as the median area CS (MACS).
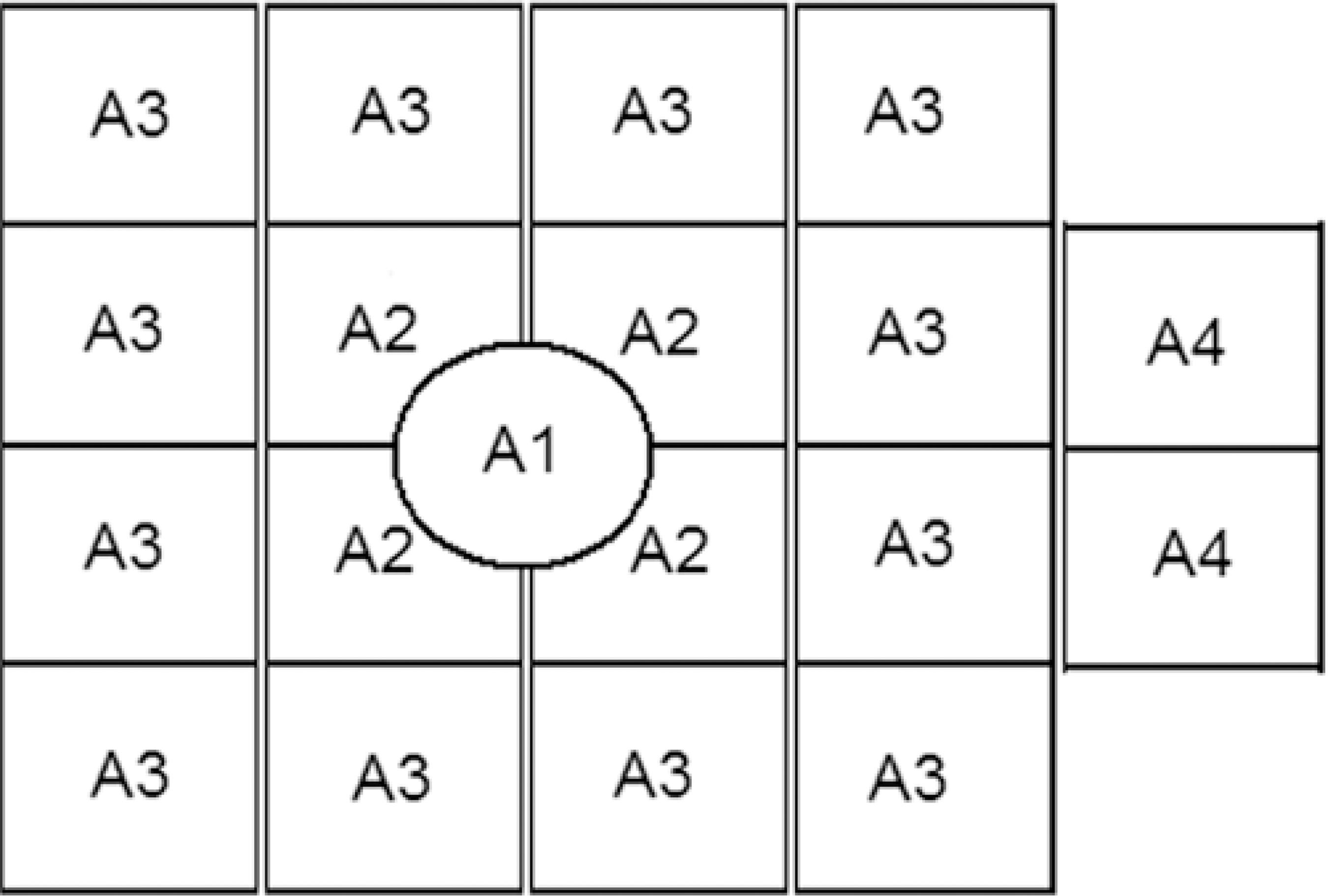
Figure 1 Representation of the frequency-doubling technology division in four areas: central (A1); paracentral (A2); peripheral (A3); and lateral (A4).
Statistical analysis was performed using SPSS for Windows (version 15; SPSS, Inc, Chicago, Illinois, USA). For primary outcome measures, P<0.05 was considered to be statistically significant. The normality of the quantitative variables was verified using the Kol-mogorov-Smirnov test. The nonparametric Wilcoxon paired test was used to compare data between the two IOL groups. The differences between MD, PSD and MACS were compared using an unpaired t test.
RESULTS
A total of 25 patients (50 eyes; 12 men [48.0%] and 13 women [52.0%]) were enrolled in the present study. The mean age of the patients was 57.80 ± 6.48 years. No significant difference was observed between the IOL groups with regard to mean IOL power (P=0.736) and mean axial length (P=0.431) (Table 1).
Table 1 Demographic characteristics of patients enrolled in the study
| Characteristics | Akreos AO | Akreos Fit | p-value |
|---|---|---|---|
| No. of eyes | 25 | 25 | |
| Age (years) | 57.80 ± 6.48 | 57.80 ± 6.48 | |
| Sex (M/F) | 12/13 | 12/13 | |
| Eye (OD/OS) | 11/14 | 14/11 | |
| IOL power (D) | 21.48 ± 2.23 | 21.51 ± 2.20 | 0.878 |
| Axial length (mm) | 22.35 ± 2.20 | 22.20 ± 2.43 | 0.630 |
M= male; F= female; OD= right eye; OS= left eye; lOL= intraocular lens; D= diopters.
No significant difference was observed between the aspheric and spherical groups for distance-corrected vision (0.00 ± 0.11 and 0.01± 0.07 logMAR, respectively) (P=0.432).
The mean pupil diameter was similar between Akreos AO and Akreos Fit under photopic (P=0.083), mesopic (P=0.066), and scotopic conditions (P=0.180) (Table 2).
Table 2 Wavefront data and pupil size of eyes implanted with Akreos AO and Akreos Fit
| Akreos AO | Akreos Fit | ||
|---|---|---|---|
| Wavefront data | (Mean ± SD) | (Mean ± SD) | p-value |
| HOARMS | 0.96 ± 0.19 | 1.39 ± 0.39 | 0.004* |
| Coma | 0.51 ± 0.19 | 0.57 ± 0.28 | 0.412 |
| Spherical aberration | 0.26 ± 0.08 | 0.45 ± 0.17 | <0.001* |
| Pupil size (mm) | |||
| Scotopic | 4.48 ± 0.46 | 4.54 ± 0.40 | 0.180 |
| Mesopic | 4.01 ± 0.45 | 4.04 ± 0.41 | 0.066 |
| Photopic | 3.48 ± 0.42 | 3.42 ± 0.40 | 0.083 |
HOA= higher-order aberration; RMS= root mean square; SD= standard deviation; mm= millimeters;
*= statistically significant.
At 12 months, the spherical equivalent was 0.03 ± 0.29 in the Akreos AO group and 0.06 ± 0.43 in other group (P=0.296). There was no significant difference between the IOL groups with regard to distance UCVA (P=0.379) and distance BCVA (P=0.331). The UCVA was 0.08 ± 0.05 in the Akreos AO group and 0.09 ± 0.06 in the Akreos Fit group. The BCVA was 0.01 ± 0.10 in the Akreos AO group and 0.02 ± 0.09 in the Akreos Fit group.
Figures 2 and 3 present the CS results. Under photopic conditions, the Akreos AO IOL was associated with significantly better CS than the Akreos Fit IOL only at the spatial frequency of 12 cycles per degree (cpd) (P=0.028). Under mesopic conditions, the Akreos AO IOL was associated with significantly better CS than the Akreos Fit IOL at all spatial frequencies (1.5, 3, 6, 12, and 18 cpd; P=0.004, P=0.042, P=0.017, P=0.0017, and P=0.001, respectively).
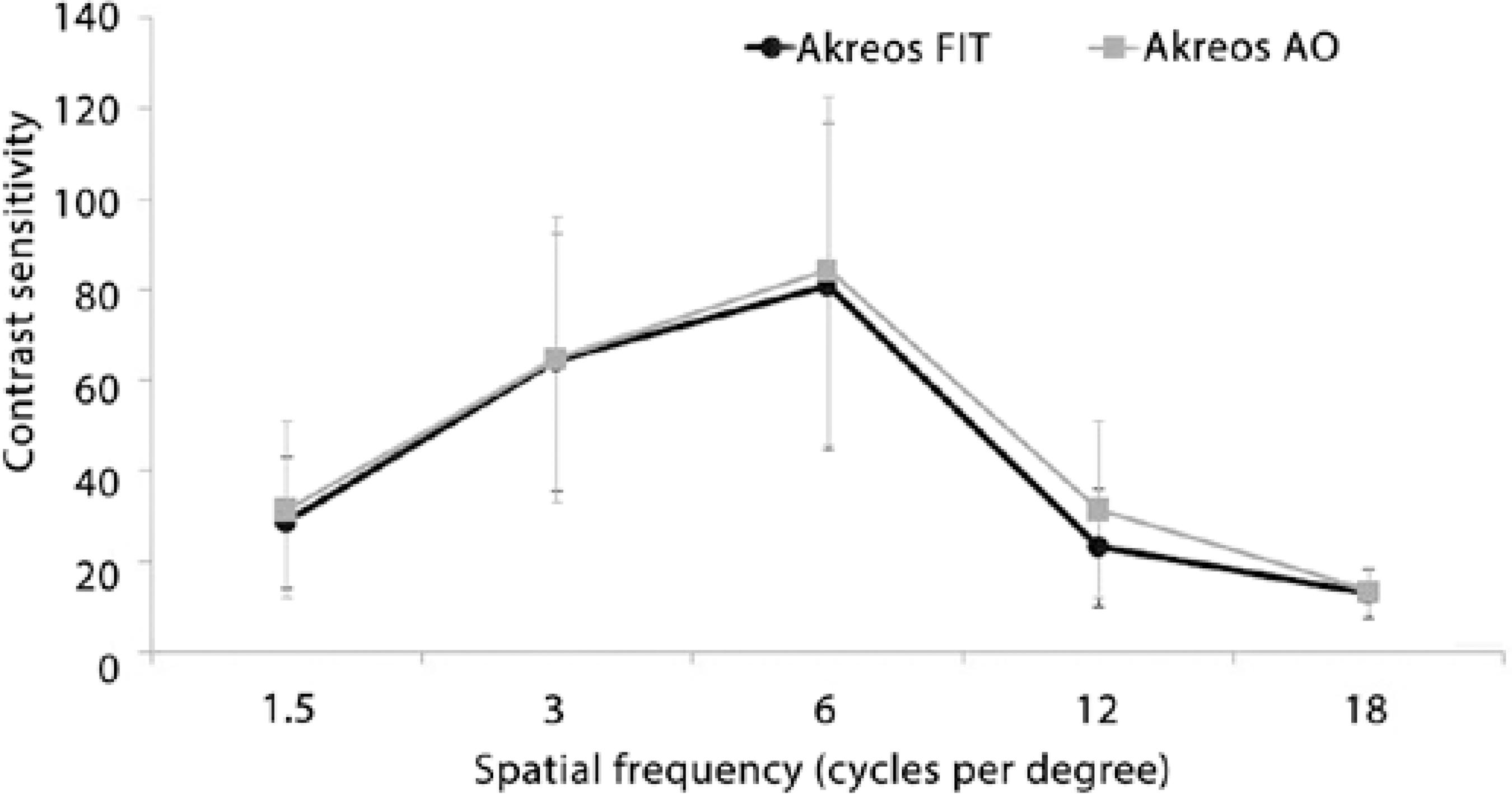
Figure 2 Graph showing contrast sensitivity under photopic conditions in the aspheric and spherical groups.
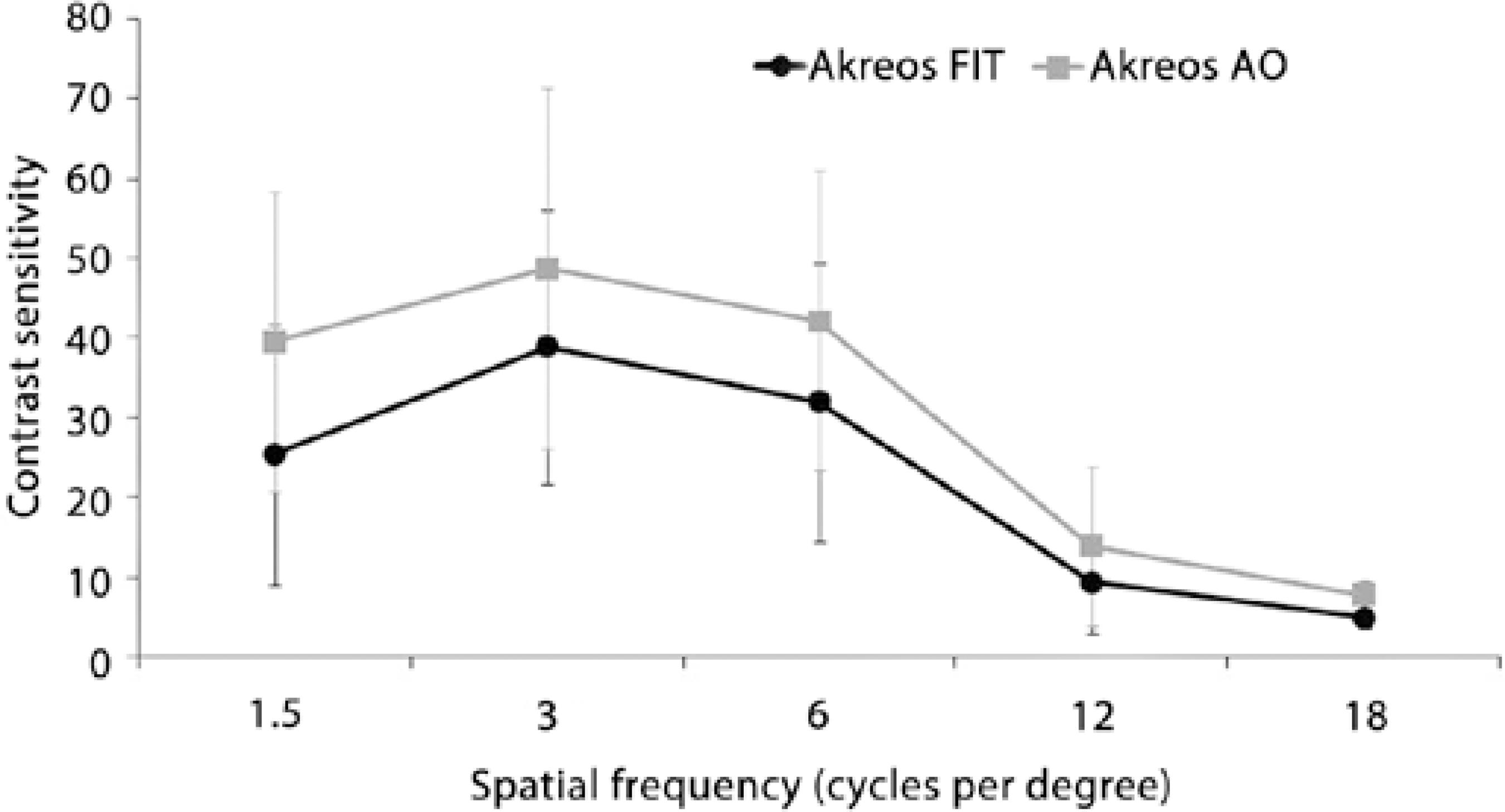
Figure 3 Graph showing contrast sensitivity under mesopic conditions in the aspheric and spherical groups.
Table 2 shows wavefront data after cataract surgery. The Akreos AO group had statistically significantly lower values of mean HOA and SA compared with the Akreos Fit group (P=0.004 and P<0.001, respectively). There were no statistically significant differences in coma values between the two IOL groups (P=0.412).
The average MDs of eyes implanted with aspheric and spherical IOLs were -1.01 ± 1.68 dB and -3.15 ± 3.78 dB, respectively, on FDT testing. There was no significant difference in either MD (P=0.051) or PSD (P=0.233) between the groups (Table 3).
Table 3 Frequency doubling technology (FDT) data of eyes implanted with Akreos AO and Akreos Fit at 12 months postoperatively
| Akreos AO | Akreos Fit | ||
|---|---|---|---|
| FDT data | (Mean ± SD) | (Mean ± SD) | p-value |
| MD (dB) | -1.01 ± 1.68 | -3.15 ± 3.78 | 0.051 |
| PSD (dB) | 5.05 ± 1.21 | 6.30 ± 3.39 | 0.233 |
MD= mean deviation; PSD= pattern standard deviation; SD= standard deviation.
Considering the VF division, the MACS of the aspheric IOL had higher values than the spherical IOL in all areas (A1 to A4), although the difference was statistically significant only in the peripherical area (A3) (P=0.043).
DISCUSSION
Functional and structural changes associated with glaucoma require special consideration in the patient who is undergoing cataract/IOL surgery. The decreased CS observed in glaucoma may be improved by the use of aspheric IOLs(18).
Contrast is one of the most important parameters activating cortical cells involved in vision processing(19). The perception of median arcuate ligament frequency doubling may be mediated by a group of retinal ganglion cells called My cells or by cortical mechanisms(20-23).
Given the similarities between the frequency-doubling illusion and CS, one might deduce that cataract extraction results in recovery of sensitivity in FDT(29). Previous studies showed that the MD of FDT and SITA-fast perimetry were improved after cataract extraction but PSD remained unchanged(24,25).
Similarly, we suspected that changes after aspheric IOL implantation may influence CS and its thresholds. Therefore, the present contralateral eye study was conducted to analyze the theoretical benefits of aspheric IOL in improving CS and its consequences in FDT parameters. To our knowledge, no previous study comparing the effect of an aspheric IOL on FDT perimetry has been published to date.
In the present study, under mesopic conditions, postoperative VCTS CS testing revealed significant differences between the two groups at all spatial frequencies, indicating that the Akreos AO IOL group performed better than the Akreos Fit group in larger pupil sizes. However, under photopic conditions, the Akreos AO IOL performed better than Akreos Fit only at 12 cpd (P=0.028). In addition, we found that the amount of HOAs was significantly lower in the aspheric group.
Although previous studies comparing aspheric and spherical IOLs have also reported the reduction of SA in patients implanted with aspheric IOLs, its influence in improved CS results is still controversial. Some studies show improvement in CS with aspheric IOLs(6-10), while others have showed no improvement(26,27).
The increase in optical quality obtained with aspheric IOLs in our study does not play a significant role in changing FDT parameters. Our results showed that both MD (P=0.051) and PSD (P=0.233) did not differ significantly between groups.
A previous study by Ueda et al.(25) reported findings that were similar to those in the present study. They analyzed the effect of clear (VA60BB, HOYA) and yellow-tinted lenses (YA60BB, HOYA) on FDT values, and concluded that both MD and PSD did not differ significantly. It is important to notice that this study was not conducted with aspherical IOLs.
Others studies have analyzed the influence of IOLs on short-wavelength automated perimetry (SWAP)(28,29). Jang et al analyzed clear (AcrySof SA60AT; Alcon Laboratories) and yellow-tinted lenses (AcrySof SN60AT or SN60WF; Alcon Laboratories) on SWAP(28). The SN60WF IOL is designed with negative SA to compensate for the positive aberration of the average cornea. The authors demonstrated that yellow-tinted IOLs may affect the SWAP results, although they did not separately analyze the effect of the aspheric IOL used (SN60WF) on perimetry.
Kara-Junior et al.(29) reported different results than Jang et al.(28).They suggested that yellow-tinted IOLs (AcrySof SN60AT) did not interfere with the results of SWAP. It should be noted that both IOLs studied generated a considerable amount of SA.
With respect to mean sensitivities of the different zones as defined in the present study, FDT testing resulted in improved CS after aspheric IOL implantation. We found higher mean values in the eye with Akreos AO in all areas (A1 to A4), but a statistical difference (P=0.043) was found only in the 20º field area (A3). Although the changes were not statistically significant, it is noteworthy that an improvement in the perimetry values with such lenses could mask the diagnosis of glaucoma or lead to other diagnostic confusions, especially considering that the functional abnormalities detected by FDT perimetry were shown to be predictive of the future onset and location of VF loss among suspected glaucoma patients(13-15).
The results of the present study indicate that MD values were higher in the Akreos AO group, and the PSD was higher in the Akreos Fit group. Note that a more negative MD indicates a worse visual performance than expected from the normative database, and a higher PSD indicates greater changes in sensibility. Previous studies in patients with yellow-tinted IOLs and patients with clear IOLs have also showed higher MD values in patients with yellow-tinted IOLs(25,28,29). However, both indices in our study were not statistically significant.
The present study had several limitations, including the absence of preoperative FDT perimetry data, the small number of cases and the absence of concurrent standard automated perimetry. A larger number of cases (approximately 100 cases in each group) could make a difference in the findings between each group, or confirm the result observed in the present study.
In addition, the present study specifically investigated these effects in patients without glaucoma. It stands to reason that in a disease process that decreases CS, an attempt to increase CS would be beneficial to the patient.
In summary, aspheric IOLs significantly reduced SA and HOAs, improving mesopic CS. Although there was a trend toward slightly improved FDT in the aspheric IOL group, it was not statistically significant. Further studies with large sample sizes should be performed to confirm or reject this hypothesis.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

