Dear Editor:
In the study of the pathophysiology for Graves ophthalmopathy (GO), a growing interest has been demonstrated in the role of nuclear receptors related to adipocyte differentiation(1), with an emphasis on peroxisome proliferator-activated receptor-gamma (PPAR-γ)(2). Another point of concern has been the inflammatory response, a result of the release by monocytes and macrophages of cytokines and other mediators, which can contribute significantly to the progression of the condition and implies that corticotherapy should be the main therapeutic approach(3). Unlike what happens in the adipocyte pathway, PPAR-γ activation has been associated with inhibition of the inflammatory process induced by certain cytokines(4), which raises doubts about the final balance of receptor stimulation regarding the evolution of GO. Furthermore, the possible relation of PPAR-γ with the treatment of an ocular condition with glucocorticoids remain to be clarified.
Here, we have reported our experience with PPAR-γ gene expression, observed in orbital fibroblast cultures from a patient with GO, in the presence of different glucocorticoid dosages. It was a white female patient, 62 years old, who had been diagnosed with Graves disease (GD) for 11 years, and was currently using methimazole (30 mg/day). She presented with inactive GO (Clinical Active Score (CAS): 2), with moderate to severe ocular involvement(3) and with bilateral proptosis (exophthalmometer reading of 24 mm and 23 mm in right and left eyes, respectively), and without strabismus or optic neuropathy. Computed tomography showed only bilateral ocular proptosis. Patient underwent orbital decompression surgery at the Clinics Hospital (CH) of the Botucatu Medical School (FMB)-Unesp. She was, at the time of the surgery, clinically controlled from the thyrotoxicosis, with serum free thyroxin levels of 1.64 ng/dL (reference ranges: 0.8-1.9 ng/dL) and thyrotropin (TSH) of 0.20 µIU/mL (reference ranges: 0.4-4.0 µIU/mL).
The fibroconjunctive material extracted from the orbital space during surgery was seeded on 199 media, with bovine fetal serum (Gibco®) and antibiotic/antimycotic solution (Sigma®); the fibroblasts resultant from this initial procedure were cultured until confluence of approximately 80%. Next they were submitted to treatment with either 10 nM or 100 nM of dexamethasone, in biological triplicate, compared for PPAR-γ gene expression, and evaluated through real time polymerase chain reaction (real time-PCR) with non-treated fibroblasts. The treatments were compared using two-factor analysis of variance (ANOVA), complemented with Tukey's multiple comparison test. The value of p<0.05 was considered statistically significant.
The fibroblasts presented a 65% greater PPAR-γ gene expression on the cells treated with 10 nM of dexamethasone compared to those not treated. The fibroblasts treated with 100 nM of dexamethasone did not differ in PPAR-γ gene expression from those who received 10nM and from those not treated (Figure 1). Thus, considering the importance of the PPAR-γ receptor to the adipocyte(1,2), one could assume that using lower doses of glucocorticoids when treating GO would imply greater gene expression and greater adipocyte differentiation in the orbital fibroblasts, with worsening of proptosis. On the other hand, PPAR-γ activation plays an inhibitory role on the inflammatory process(4 ). From this point of view, lower glucocorticoid doses would imply a smaller PPAR-γ-mediated inflammatory response. However, there has been evidence suggesting that the anti-inflammatory potential of this receptor activation would not be as significant as that of other receptors from the same family, such as PPAR-α(4). Therefore, considering the preponderant action of PPAR-γ on adipogenesis, when opting for glucocorticoids in the treatment of GO, we could speculate that the ideal would be not using low doses, which would stimulate expression of the receptor, increasing the adipocyte differentiation and contributing to progression of the exophthalmos. Thus, our experience here could suggest that, for the GO therapeutic approach, higher doses of glucocorticoids would be more adequate. However, our data did not support this conclusion, since the expression of the receptor in cells treated with 100 nM dexamethasone was similar to untreated cells, and was not higher. Moreover, avoiding low doses of glucocorticoids based on one presumptive action of the PPAR-gamma receptor would be not acceptable due to the other many actions of corticosteroids on the GO process. Thus, we have concluded that none of the treatments tested in this ex vivo study proved superior for the reduction of mRNA expression of PPAR-γ. Furthermore, as only one patient's data has been evaluated, as it may be difficult to extrapolate the dosages from cell culture to clinical practice and, considering the risks reported in patients treated with high doses, more studies will be needed in order to quantify the ideal dosage of glucocorticoids on the treatment of GO.
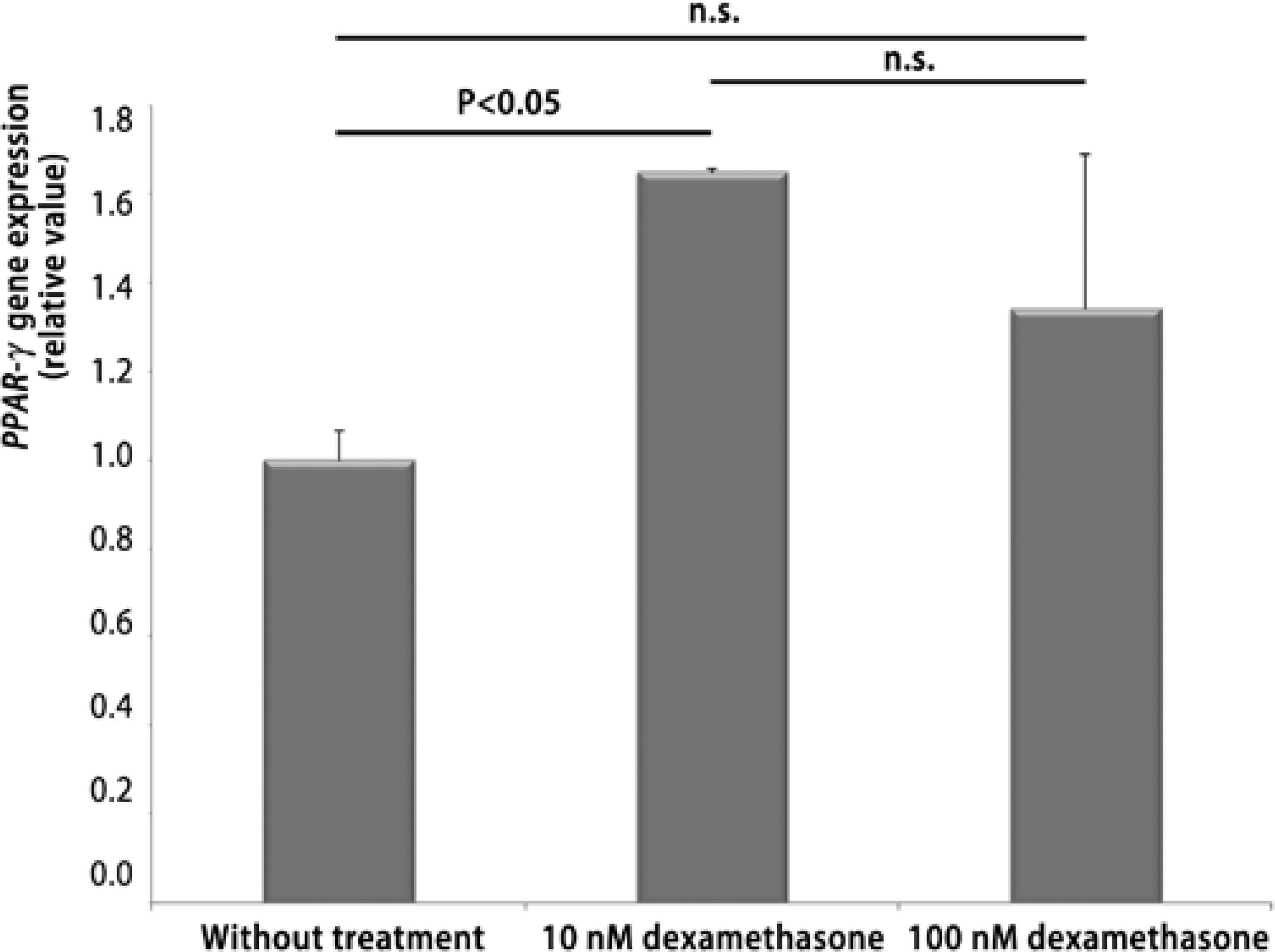
Figure 1 Effect of dexamethasone, in doses of 10 and 100 nM, on peroxisome proliferator- activated receptor-gamma (PPAR-γ) gene expression on the orbital fibroblasts of the patient with Graves ophthalmopathy. Data is expressed in mean and standard-deviation, and compared using ANOVA complemented with Tukey's test. Significant values were represented by p<0.05.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

