INTRODUCTION
Eye burns are common and may be caused by various chemical and physical agents including acids, alkalis, high temperatures, and fire(1). They are most generally a consequence of chemical handling accidents and may result in permanent damage to the ocular surface and visual function(1).
Corneal alkali burns are considered an ophthalmologic emergency. Therefore, timely recognition and implementation of the appropriate treatment represent important steps in controlling the progression of early and late complications(2). The literature describes various forms of treatment for corneal alkali burns. These include artificial tears, collagenase inhibitors, therapeutic contact lenses, topical fibronectin, topical vitamin C, topical citrate(2), conjunctival transplantation(1), amniotic membrane patching(3,4), limbal transplantation(5), and autologous serum eye drops(6) as well as treatment of the severe inflammatory processes with topical or systemic corticosteroids.
Chemical burns may lead to devastating complications, including corneal perforation due to the rapid degradation of collagen fibers(1). Recently, riboflavin-ultraviolet-A-induced cross-linking (CXL) was developed as a technique for enhancing collagen cross-linking in the treatment of corneal wounds(7). CXL has been reported to be a safe and effective method for controlling the progression of corneal ectasia(8,9). The procedure stops the melting process of the cornea(10)and has been shown to increase the resistance of porcine corneas by inhibiting enzymatic degradation(11). CXL was previously used in five cases of corneal necrosis following a bacterial infection refractory to clinical treatment(12), and it was demonstrated that this technique constitutes a useful alternative to emergency keratoplasty by increasing corneal resistance against the action of collagenolytic enzymes. Currently, CXL is used to stabilize degenerative corneal disorders such as corneal ectasia (in keratoconus patients and following refractive surgery), where it acts to increase the degree of rigidity of the stromal collagen fibers(13,14).
Considering these aspects, the objective of the present study was to evaluate the effect of CXL in a rabbit model, initiated one hour after a corneal alkali burn.
METHODS
This was a comparative, randomized, blinded experimental study conducted in the animal laboratory and the animal experimentation surgical center of the Santa Casa de São Paulo. The institutional Animal Research Ethics Committee of UNIFESP and the Santa Casa de São Paulo approved the protocol (approval letter 1476/09). The animal study was carried out in compliance with the recommendations of the Association for Research and Vision in Ophthalmology (ARVO).
Animal selection
Ten male New Zealand white rabbits weighing 3-4 kg were randomly divided into two groups, with five rabbits in each group (the experimental and control groups). The animals were provided with food and water ad libitum and pertinent veterinary supervision.
Experimental model of corneal alkali burn
An experimental model of corneal burn that has already been described in previous studies was used(15,16). This model was modified only with respect to the diameter of the paper disc in order for it to include the total limbal area. A grade IV ocular burn, according to the Roper Hall classification(17,18), was induced in the right eye of all the rabbits.
Under the supervision of a veterinarian, general anesthesia was induced by an intramuscular injection of ketamine hydrochloride (25 mg/kg of weight) associated with 2% xylazine hydrochloride (4 mg/kg of weight). Appropriate ventilatory support was provided. Corneas were anesthetized using a drop of 1% proparacaine hydrochloride ophthalmic drops in the animals' right eyes. A corneal alkali burn was then induced by applying a 14 mm diameter filter paper disc (Whatman filter paper, # 40) soaked in 1N NaOH (Figure 1). This disc was maintained in contact with the cornea and the limbus for one minute, after which the eyes of the animals were washed with 1.0 cc of 0.9% NaCl.
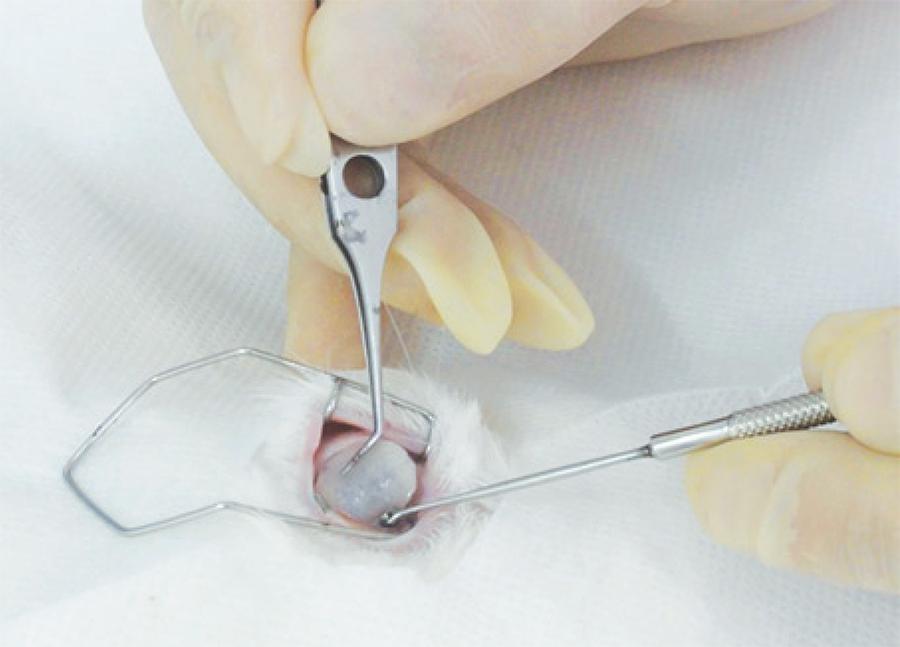
Figure 1 Induction of ocular burns with alkali-immersed filter disks: 14 mm diameter filter paper discs (Whatman filter paper, # 40) soaked in 1 N NaOH.
Sixty minutes after the injury procedure, 0.1% riboflavin drops (Ophthalmos®, São Paulo, Brazil) were applied every five minutes for thirty minutes to the animals in the experimental group. This step was then followed by irradiation with ultraviolet light (UVA 370 nm, with an irradiance of 3 mW/cm2 and a surface dose of 5.4 J/cm2; X-link, Opto, Brazil) for 30 minutes at a distance of 45 mm from the right eye, and including the total cornea and limbus area. The epithelium was not touched during the procedure. During UVA irradiation, 0.1% riboflavin drops continued to be applied concomitantly every five minutes. Next, clinical treatment was initiated with the application of a 1% prednisolone acetate ophthalmic suspension (Pred Fort, Allergan, Guarulhos, SP, Brazil), preservative-free lubricant eye drops (Optive UD, Allergan), and 0.3% ofloxacin eye drops (Oflox, Allergan). These were all administered three times a day (tid) for the first 15 days (early phase). After this period, the corticosteroid treatment was stopped and only the lubricant eye drops and the antibiotic were maintained for another 15 days (regenerative phase).
The control group (5 eyes in 5 rabbits) was submitted to the same clinical treatment described above after the burn had been induced and the eye washed with 1.0 cc of 0.9% NaCl.
Follow-up and examination of the animals
The animals underwent a 30 day follow up, with evaluations being conducted by two independent, blinded observers immediately after the burn, and then on days 1, 7, 15, and 30 following the injury. Evaluation of the corneal surface was conducted by external examination and then by biomicroscopy using a hand-held slit lamp (Heine-HSL 100, USA).
Thirty days after the ocular burn, all the rabbits were sacrificed using a 3 ml intramuscular injection of 0.2% acepromazine, followed 15-20 minutes later by 5 ml intravenous thionembutal and 3 ml intravenous potassium chloride. The corneas were then excised and histological sections were prepared and examined following hematoxylin/eosin (HE) and trichrome Masson staining for assessing the collagen stroma in the two groups.
Clinical parameters used
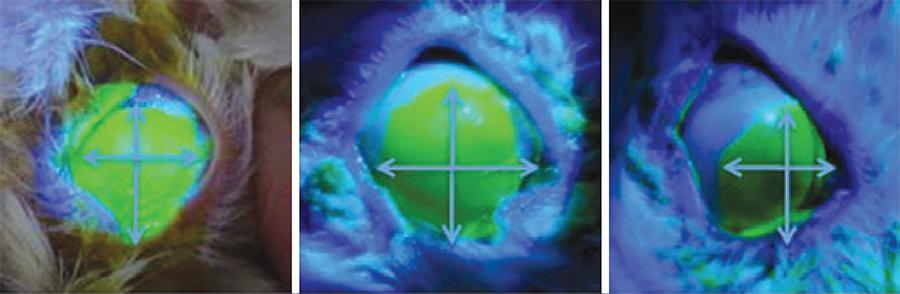
Clinical parameters are described on table 1(16,18-20). An example of epithelial defect evaluation can be observed on figure 2.
Table 1 Grading of clinical parameters evaluated
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Secretion(18) | Absent | Traces of secretion in the cul-de-sac or eyelid margins | Small amount of secretion visible in the conjunctiva and on the eyelid margins | Large amount of secretion, eyeball is still visible | An excessive amount of secretion, hampering the opening of the palpebral fissure |
| Hyperemia and chemosis(18) | Absent | Slight conjunctival injection | Moderate conjunctival injection with mild chemosis | Moderate to intense injection of vessels, mild chemosis | Intense injection of vessels, intense chemosis |
| Neovascularization | Absent | One vessel extending 0.5 mm from the limbus | One or more vessels, with fewer than five branches, extending 0.5 mm from the limbus | Three or more vessels; or two vessels, with more than five branches, extending 0.5 mm from the limbus | |
| Ciliary injection(19,20) | Absent | <1 mm | 1-2 mm | >2 mm | |
| Corneal edema(19,20) | Absent | Present, details of the iris are visible | No details of the iris are visible | Neither the iris nor the pupil is visible | |
| Limbal ischemia(16) | Absent | Less than one-third of the limbus circumference | Between one-third and half of the limbus circumference | More than half the limbus circumference | |
| Blepharospasm | Absent | Present | |||
| Symblepharon | Absent | Present | |||
| Epithelial defect | Mean between the largest horizontal and the largest vertical measurements of the fluorescein-stained epithelial defect, as measured in millimeters using a surgical caliper. (Figure 2) | ||||
| Corneal injury | Mean between the largest horizontal and the largest vertical measurements of the opacity lesion created by the burn, measured in millimeters using a surgical caliper | ||||
Statistical analysis
Kappa coefficients were calculated to estimate observer agreement for all the clinical variables investigated. Fisher's exact test was used to compare differences between the experimental and control groups. For the continuous variables, means, standard deviations, medians, and ranges were calculated. The Mann-Whitney U test was used to compare the continuous variables between groups, with p values <0.05 being considered statistically significant. The complete statistical analysis was conducted using the STATA statistical software package, version 10 (College Station, Texas, USA).
RESULTS
There was high agreement between the two independent observers, as shown by the kappa coefficient range of 0.32-1.0.
There was a statistically significant difference between the two groups with respect to the mean size of the corneal epithelial defect at 15 and 30 days after the injury procedure (Table 2). There was also a statistically significant difference in the mean extent of corneal injury (opacity lesion) at day 30 (Table 3).
Table 2 Mean size of the corneal epithelial defect in the rabbits following ocular alkali burn
| Time post-injury | Size of the corneal epithelial defect (Mean ± SD) (mm) | p-value* | |
|---|---|---|---|
| Experimental group | Control group | ||
| 1st day | 13.20 ± 0.20 | 13.20 ± 0.20 | 1.000 |
| 7th day | 9.85 ± 0.87 | 10.95 ± 0.71 | 0.089 |
| 15th day | 6.40 ± 0.92 | 9.10 ± 0.87 | 0.008 |
| 30th day | 4.05 ± 0.94 | 6.40 ± 0.65 | 0.008 |
Experimental group= cross-linking plus clinical treatment; Control group= only clinical treatment;
P*= Mann-Whitney U test, with p-values <0.05 being considered statistically significant.
Table 3 Mean extent of the corneal injury (opacity lesion) in the rabbits after ocular alkali burn
| Time post-injury | Extent of the corneal injury (Mean ± SD) (mm) | ||
|---|---|---|---|
| Experimental group | Control group | p-value* | |
| 1st day | 13.10 ± 0.22 | 13.00 ± 0.11 | 0.881 |
| 7th day | 13.05 ± 0.00 | 12.95 ± 0.11 | 0.317 |
| 15th day | 12.20 ± 0.44 | 12.00 ± 0.58 | 0.737 |
| 30th day | 10.35 ± 0.74 | 11.60 ± 0.54 | 0.021 |
Experimental group= cross-linking plus clinical treatment; Control group= only clinica treatment;
P*= Mann-Whitney U test, with p-values <0.05 being considered statistically significant.
No statistically significant differences were found between the experimental and control groups with respect to clinical parameters such as conjunctival hyperemia, ocular secretion, corneal neovascularization, ciliary injection, central corneal edema, limbal ischemia, blepharospasm, or symblepharon during the post-injury followup (Table 4).
Table 4 Mann-Whitney U-test p-values for the various clinical parameters evaluated at the different time-points following the injury procedure
| Clinical parameters | Time post-injury | |||
|---|---|---|---|---|
| (p-values) | 1st day | 7th day | 15th day | 30th day |
| Ocular secretion | 0.206 | 0.444 | 0.444 | 0.444 |
| Conjunctival hyperemia | 0.444 | n/c | 1.000 | 0.167 |
| Neovascularization | n/c | 0.167 | 0.524 | 0.524 |
| Ciliary injection | 0.444 | 0.167 | 0.444 | 1.000 |
| Edema | 1.000 | 1.000 | 1.000 | 1.000 |
| Limbal ischemia | n/c | n/c | n/c | n/c |
| Blepharospasm | n/c | n/c | n/c | n/c |
| Symblepharon | n/c | n/c | 0.206 | 0.206 |
P*= Mann-Whitney U test, with p-values <0.05 being considered statistically significant; n/c= not calculated.
In the CXL group, histology revealed collagen bridges linking collagen fibers that were arranged in an organized pattern (Figure 3). However, these bridges were absent in the control group (Figure 4). Table 5 shows the presence or absence of bridges in the corneas evaluated.
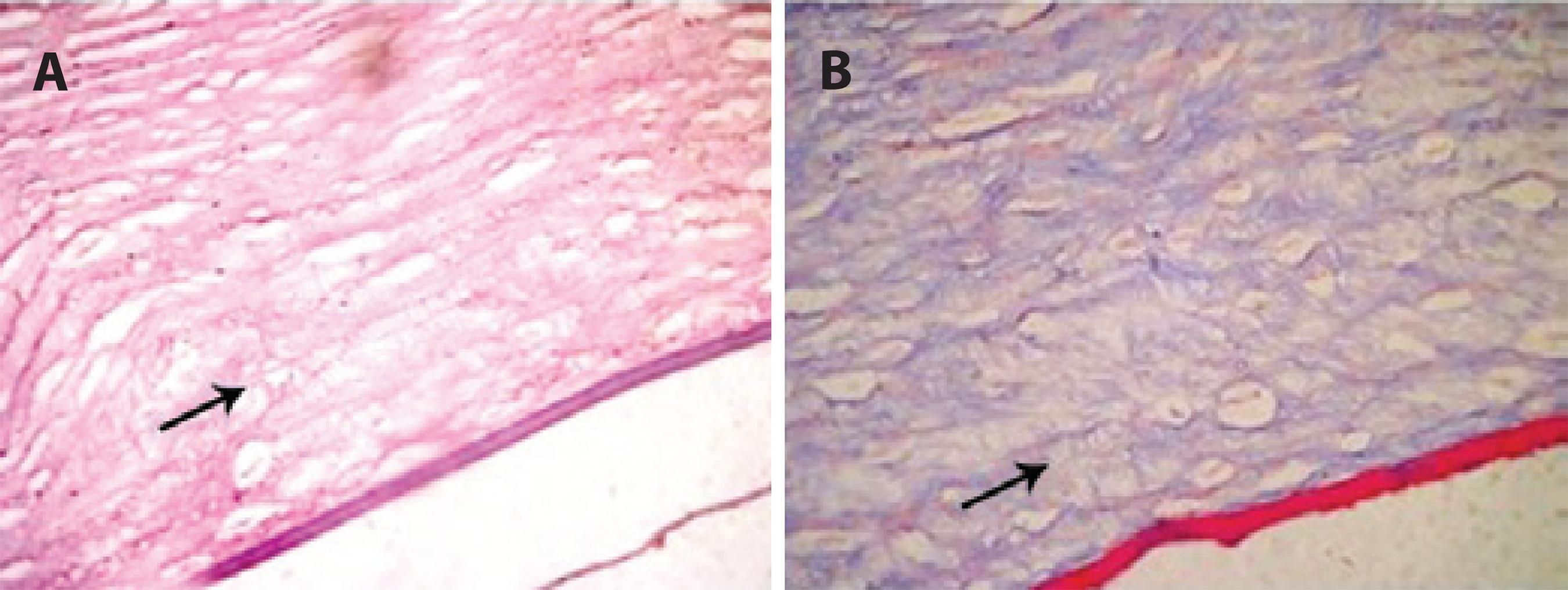
Figure 3 Alkali-burned corneal stroma following 30 days of CXL treatment. Note the organized pattern of collagen bridges (arrow) between the stromal fibers. HE staining (A) and trichrome Masson staining (B).
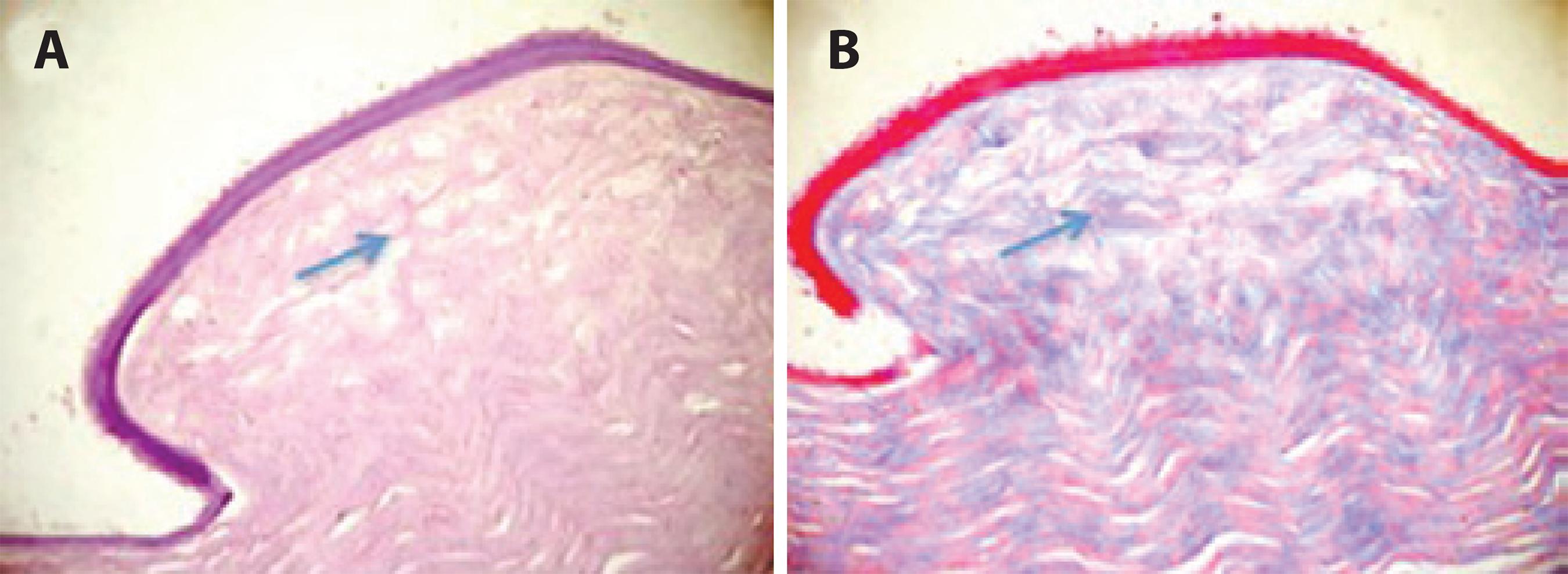
Figure 4 Corneal stroma 30 days after the alkali-burn procedure in the control group. Note the disorganized pattern of collagen fibers and the absence of bridges (arrow) between the stromal fibers. HE staining (A) and trichrome Masson staining (B).
Table 5 Presence or absence of stromal interfibrillar bridges in histological sections of alkali-burned rabbit corneas according to the procedure performed (CXL or none)
| Animal number | Corneal procedure | Interfibrillar bridges |
|---|---|---|
| 1 | CXL | Yes |
| 2 | CXL | No |
| 3 | CXL | Yes |
| 4 | CXL | Yes |
| 5 | CXL | No |
| 6 | Control | No |
| 7 | Control | No |
| 8 | Control | No |
| 9 | Control | No |
| 10 | Control | No |
DISCUSSION
In the present study, we used 14 mm alkali-immersed filter discs (1 N NaOH) to create severe ocular burns (Roper Hall criteria, grade IV). This methodology was chosen because it has been well described and involves clinically comparable parameters that can be easily measured.
We hypothesized that CXL would allow covalent bonds to be formed between the collagen fibrils, thus promoting thickening of the collagen fibrils through the deposit of structural molecules such as proteoglycans(21), and possibly making the cornea more resistant to the effect of collagenolytic enzymes. In the CXL group, histopathological examinations revealed collagen bridges linking the collagen fibers in the corneal stroma. This may indicate that the collagen fibers in the CXL group were more resistant to collagenolytic enzymes than those in the control group. Furthermore, the arrangement pattern of the stromal collagen fibers was more organized in the CXL group compared to the control group. This may also indicate a greater resistance of the collagen fibers to collagenolytic enzymes in the CXL group, resulting in improved wound healing. Nevertheless, further investigation is required with respect to these collagen bridges and wound healing. Some case reports have also shown the antimicrobial effect of ultraviolet light associated with riboflavin in the treatment of infectious keratitis. The mechanism is presumably either due to the bactericidal effect of ultraviolet light or to the increased resistance of the collagen fibers of the cornea, which prevents the infectious agent from proliferating(12,21,22).
A statistically significant difference was found between the groups regarding the mean extent of the corneal injury on day 30 following the injury (p=0.021). At this time point the lesion caused by the ocular burn is at a late phase of tissue repair; the effect of collagenolytic enzymes has been largely overcome, and the tissue has undergone regeneration of the fibroblasts, with migration of myofibrils to the site and progressive re-epithelization taking place. The associated effects of the increase in resistance to the collagenases in the first few weeks, and the increase in the rigidity of the corneal tissue due to cross-linking, suggests a better recovery in the CXL group compared to the control group. It should be noted that even considering the statistical significance of our results with respect to the mean extent of the corneal injury, when the absolute data is taken into consideration the difference in the mean size of the lesions is close to 1 mm.
The magnitude of this measurement is imperceptible in clinical practice when considering devastating grade IV burns.
Statistically significant results regarding the mean size of the corneal epithelial defect were also found on post-injury days 15 (p=0.008) and 30 (p=0.008). Therefore, we can conclude that the centripetal movement of the corneal epithelial cells that occurs in the regeneration phase of the burned cornea occurred faster in the experimental group compared to the control group. The centripetal movement is associated with an increase in the rigidity of the cornea and the resistance of the tissue to enzymatic degradation through the formation of stronger stroma. This serves as a base for the sliding of the epithelial cells during the recovery phase of the corneal epithelial wound.
Some of the difficulties encountered during this study that merit particular mention refer to the objective measurement of the data obtained from the rabbits, and the manipulation of the animals' eyes when using a hand-held slit lamp. Measuring the greatest horizontal and vertical diameters also proved difficult, since although the lesions were circular and symmetrical throughout their extension, in some cases there were slight discrepancies in re-epithelialization. This occasionally gave rise to asymmetry in the shape of the healing wound. In addition, the sample size used in this study was small (5 rabbits in the CXL group and 5 in the control group). However, it is important to stress that these numbers were agreed upon following an in-depth discussion with the ethics committee for animal research.
The mechanism underlying the statistically significant differences found at later post-injury time-points remains largely unclear, and we therefore recommend that further studies be conducted to clarify the physiopathogenesis of cross-linking in ocular burns.
In conclusion, these results suggest that the use of CXL may improve the prognosis of acute corneal alkali burns.




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

