INTRODUCTION
Pterygium is a common ocular surface disease in countries situated near the equator and is characterized by corneal epithelial overgrowth, usually occupying a nasal interpalpebral location(1). The pathogenesis of pterygia seems to be multifactorial, and the variable presence of human papillomavirus suggests that it is not a required factor for lesion development(2). Epidemiological evidence suggests that chronic sunlight exposure without ultraviolet (UV) radiation protection a crucial role(3). Pterygium growth and development seems to be associated with the proliferation of epithelium(4).
Ocular surface squamous neoplasia (OSSN) is the most common tumor of the ocular surface. Its spectrum ranges from mild to severe dysplasia, and through full thickness epithelial involvement to invasive squamous cell carcinoma. OSSN aetiology is multifactorial, but exposure to excessive amounts of solar UVB radiation is recognized as a major risk factor(5). OSSN is known to masquerade as scar tissue or pannus, and can appear in association with pterygia. Because OSSN and pterygia share risk factors, it is not surprising that they often occur concomitantly in the same eye. As shown in table 1, the incidence of coexistent pterygia and ocular surface disorders such as OSSN varies depending on the geographic location(6-15). The clinical characteristics of pterygia patients who have unsuspected OSSN does not appear to differ from those who are free of the condition. Thus, it is difficult to diagnose these patients based on clinical characteristics alone(15).
Table 1 Premalignat ocular surface disease in association with pterygium reported after histopathological study
| References | Publication year | Study population | Sample size | Associated ocular disease | % |
|---|---|---|---|---|---|
| Sevel and Sealy(6) | 1969 | Cape Town | n=100 | OSSN | 29.0 |
| Clear et al.( 7) | 1979 | Malawi | n=234 | OSSN | ~30 |
| Perra et al.(8) | 2006 | Ecuador | n=80 | PAM with atypia | 2.5 |
| Hirst et al.(9) | 2009 | Brisbane | n=533 | OSSN | 9.8 |
| Chui et al.(10) | 2011 | Sydney | n=100 | OSSN | 5.0 |
| PAM with atypia | 1 | ||||
| Yeung et al.(11) | 2011 | Toronto | n=1127 | OSSN | 0 |
| Leite(12) | 2011 | Campo Grande | n=43 | OSSN | 39.5 |
| Galor et al.(13) | 2012 | Florida | n=384 | OSSN | 4.1 |
| Oellers et al.(14) | 2013 | Florida | n=2005 | OSSN | 1.7 |
| Artornsombudh et al.(15) | 2013 | Bangkok | n=498 | OSSN | 1.8 |
OSSN= ocular surface squamous neoplasia; PAM= primary acquired melanosis.
Impression cytology (IC), a method for the evaluation of superficial cell layers in the diagnosis(16) and treatment follow-up of several ocular surface tumors(17), has exhibited squamous metaplasia with goblet cells in specimens obtained from pterygia(18-19). A graded series of ocular surface changes has been described throughout the bulbar conjunctiva, with the most advanced changes occurring directly over the pterygium surface, confirming that pterygium is indeed an ocular surface disorder(18). To the best of our knowledge, there has been no previous report regarding the use of IC for detecting unsuspected OSSN cells in pterygia. This was therefore the purpose of the present study.
METHODS
A transversal prospective observational study was conducted at the Department of Ophthalmology, Universidade Federal de São Paulo, Brazil, after approval by the Medical Ethics Committee of the institution. A complete informed consent form was obtained from all patients. Thirty-two Brazilian patients with a clinical diagnosis of primary pterygia, all of whom underwent pterygium surgery during a single day, were included. None had any clinical suspicion of OSSN. No patient received topical treatment prior to IC treatment. Clinical diagnosis was based on slit-lamp biomicroscopy. Demographic data, including gender, race, and age were recorded.
After a complete ophthalmologic examination, IC samples were harvested from lesions. In brief, after administration of topical anesthesia with 0.5% proximetacaine hydrochloride (Anestalcon ® 0.5%, Alcon, Sao Paulo, Brazil), a strip of acetate cellulose filter paper with a pore size of 0.45µ (Millipore HAWP304F0, Bedford, EUA) was placed onto the surface of the patient’s lesion. Pressure was gently applied for 5 seconds and it was then peeled off. A second sample was obtained from the same area. Filters were immediately fixed for approximately ten minutes in a solution containing glacial acetic acid, 37% formaldehyde, and ethyl alcohol at a 1:1:20 volume ratio. All samples were processed for periodic acid-Schiff (PAS), Gill’s hematoxylin, and Papanicolaou staining according to a previously published technique(1616). From the slide sets, only IC specimens that demonstrated at least one-third of the filter surface to be filled with epithelial cells were evaluated by optical microscopy, with consensus by two cytologists (J.N.B, M.S.L). The analysis included an assessment of epithelial cell morphology and a search for inflammatory cells. Atypical cells suggestive of OSSN were identified by the presence of nuclear enlargement, hyperchromasia, irregular nuclear outline, and coarse nuclear chromatin, under magnifications of 100, 200, and 400x, according to an established definition(16).
All patients underwent excision on the same day as IC sampling. Surgical removal was performed by multiple surgeons. Specimens were formalin fixed, paraffin embedded, and hematoxylin-eosin stained. They were then submitted to histopathogical study, in which consensus existed regarding the diagnoses of two experienced ocular pathologists (M.S.L, M.C.M). These two pathologists performed all optical microscopy analyses in a masked fashion. Lesions were classified according to the histopathological criteria previously published for epithelial lesions of the conjunctiva(20). Histopathology was considered to be the gold standard methodology. The sensitivity, specificity, and positive and negative predictive values were calculated, along with their respective 95% confidence intervals. All statistical analyses were performed using SPSS software (version 11.5 for Windows; SPSS, Inc., Chicago, Illinois, EUA).
RESULTS
Demographic data are presented in table 2. Patient ages ranged between 28 and 81 years, with a median of 49 years. Seventeen patients (53%) were male and fifteen (47%) were female. Regarding race, 22 (69%) were white and ten (31%) were black. Lesions were located on the nasal side in all cases. Histopathological diagnosis was pterygium without atypical cells in 19 cases (59.4%) and pterygium with unsuspected and associated OSSN cells in 13 cases (40.6%). Examples are given in figures 1 and 2. All 32 lesions showed elastosis.
Table 2 Characteristics of patients with clinical diagnosis of pterygia
| Case | Gender | Race | Age | Atypical cells on IC | Atypical cells on histology |
|---|---|---|---|---|---|
| 1 | M | W | 50 | Y | Y |
| 2 | M | B | 38 | N | N |
| 3 | F | B | 51 | N | N |
| 4 | M | B | 51 | Y | Y |
| 5 | F | B | 42 | N | N |
| 6 | M | W | 58 | N | N |
| 7 | M | W | 47 | Y | Y |
| 8 | F | W | 50 | Y | Y |
| 9 | M | W | 28 | N | N |
| 10 | M | W | 30 | Y | N |
| 11 | M | B | 33 | Y | Y |
| 12 | F | B | 32 | Y | Y |
| 13 | F | W | 59 | Y | Y |
| 14 | M | B | 56 | Y | Y |
| 15 | M | B | 34 | N | N |
| 16 | M | W | 52 | Y | Y |
| 17 | F | B | 47 | N | N |
| 18 | M | W | 67 | N | N |
| 19 | M | W | 48 | N | N |
| 20 | F | B | 60 | N | N |
| 21 | F | W | 51 | N | Y |
| 22 | F | W | 46 | N | N |
| 23 | M | W | 37 | Y | Y |
| 24 | F | W | 81 | N | N |
| 25 | F | W | 55 | N | N |
| 26 | M | W | 39 | N | N |
| 27 | F | W | 61 | N | N |
| 28 | F | W | 41 | N | N |
| 29 | M | W | 66 | N | N |
| 30 | F | W | 55 | N | N |
| 31 | F | W | 62 | Y | Y |
| 32 | M | W | 48 | Y | Y |
M= male; F= female; W= white; B= black; Y= yes; N= no; IC= impression cytology.
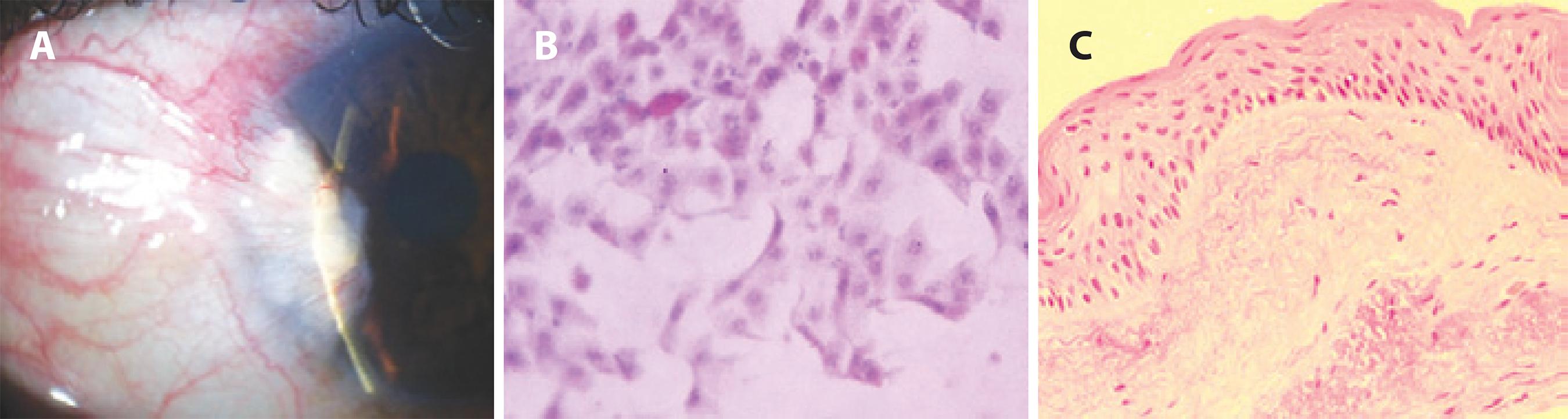
Figure 1 A) Example of an anterior segment slit-lamp photograph demonstrating a conjunctival and corneal lesion, consistent with a diagnosis of preoperative pterygium. B) Impression cytology image of a goblet cell and conjunctival epithelial cells without nuclear atypia (PAS, hematoxylin and eosin stain, original magnification, ×200). C) Photomicrograph of the histopathologic specimen from this excised lesion revealing a mucosal epithelium with appropriate maturational sequencing throughout the tissue (hematoxylin and eosin stain, original magnification, ×200).
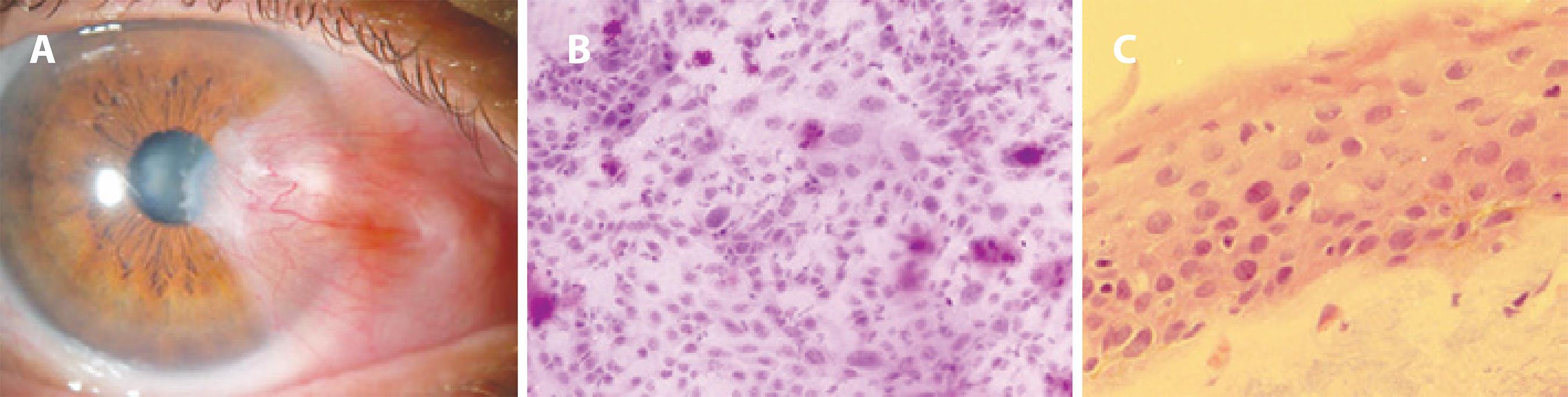
Figure 2 A) Anterior segment slit-lamp photograph of preoperative pterygium with clinically unexpected OSSN. B) Impression cytology disclosing foci of atypical epithelial cells among cells with normal morphology and goblet cells (PAS, hematoxylin and eosin stain, original magnification, ×200). C) Photomicrograph of the histopathologic specimen from this excised lesion confirming atypical epithelial cells within the epithelia (hematoxylin and eosin stain, original magnification, ×400).
Regarding the 13 subjects who had pterygia-associated OSSN, as diagnosed by histopathology, the mean age at presentation was 48 years (range, 32-62 years). Eight (62%) were male and five (38%) were female. Nine (69.2%) were white and four (30.8%) were black. In the OSSN specimens, atypia was intraepithelial in all cases. For the 19 patients without atypical cells, the mean age was approximately 50 years (range, 28-81 years).
IC demonstrated one false-negative and one false-positive result. Statistical analysis of IC performance showed 92% sensitivity, 94% specificity, a positive predictive value of 92%, and a negative predictive value of 94% (Table 3).
Table 3 Specificity, sensibility, positive and negative predictive values and prevalence of lesions
| Confidence interval (95%) | ||||
|---|---|---|---|---|
| Parameters | Estimated values | Standard error | Inferior limit | Superior limit |
| Specificity | 0.947 | 0.051 | 0.740 | 0.999 |
| Sensibility | 0.923 | 0.074 | 0.640 | 0.998 |
| Positive predictive value | 0.923 | 0.074 | 0.640 | 0.998 |
| Negative predictive value | 0.947 | 0.051 | 0.740 | 0.999 |
| Prevalence | 0.406 | 0.087 | 0.237 | 0.594 |
DISCUSSION
Here, we present a study in which 32 patients with clinically diagnosed pterygia had IC samples obtained from ocular surface cells prior to pterygium surgery and subsequent histopathological examination. The detection of unsuspected atypical epithelial cells by IC was closely correlated with detection using the gold standard histopathological examination. Bias was reduced by masking the cytologists and pathologists. Previous studies of IC samples obtained from pterygia surfaces have reported conjunctival squamous metaplasia and goblet cells without atypical cells(18-19). However, IC analysis of subclinical intraepithelial OSSN has already been described. In this study, the cytologic pattern of OSSN without any clinically visible abnormality differed from that seen in eyes with a clinically detectable disease, because the dysplastic cells were often few and they were found within sheets of normal epithelium(21). This is consistent with to our findings and we speculate that OSSN cells may arise from pterygia as part of a continuous disease spectrum(14).
Analysis of the demographic data showed that the patients with unsuspected OSSN cells showed a similar mean age to patients with simple pterygia. This is in contrast to a previous report from another geographic location that found that OSSN occurred in older patients(14). In our sample, all lesions were located on the nasal side and all cases of OSSN cells were unexpected, whereas in the previous report, there was a significant predominance of OSSN cells in the inferior location. It has been suggested that the suspicion of malignancy should be increased when a lesion does not present at the nasal or temporal position(14).
In the current study, IC analysis resulted in one false-negative and one false-positive result. The false-negative result for cytological atypia occurred in a patient whose IC showed cellularity limited to one-third of the filter paper in the two obtained samples. There were also abundant goblet cells and mucin imprints overlaying the epithelial cells in which atypia was potentially present. The false-positive result probably occurred because of the presence of an intraepithelial inflammatory process at the pterygium surface, and this appears to have produced a diagnostic dilemma for the two cytologists. After this false-positive case was reviewed, both cytologists agreed that the atypia could be secondary to inflammation occurring in the pterygium. This was because although there was some nuclear enlargement, the nucleus contour was predominantly regular and the chromatin was uniform and differed from the OSSN atypical cells, which showed an irregular nuclear outline and coarse nuclear chromatin(16).
In our sample series, elastosis (histopathological evidence of solar injury) was observed in all patients, confirming sun damage to the ocular tissue. This finding helps explain the rate of OSSN found in pterygia tissue, since sunlight exposure is a common etiological factor in both diseases(9). McKelvie et al. have reported elastosis in 100% of their Australian OSSN specimens(22), while a previous Brazilian study found it in 81.4% of OSSN samples(12), and Tabrizi et al. found it in 50% of their cases(23). Elastosis is a pathological manifestation of actinic damage to the tissue, where collagen, ground substance, and fibrocytes are altered to form an abnormal elastotic material. It can be seen in a routine haematoxylin and eosin stain as blue-grey, wavy, and irregular sized strands in the subconjunctival areas. This material changes its color to dark brown after elastic staining. According to Tulvatana et al., sun exposure, as demonstrated by pathological solar elastosis, is also one of the risk factors for the development of conjunctival squamous cell neoplasia(24).
The early detection of the premalignant stages of OSSN plays an important role in treatment success(25). However, no data currently exists to indicate whether subclinical and unsuspected OSSN will result in clinical disease; i.e., it is not possible to demonstrate an absolute correlation between the histopathological abnormality and the biological potential of the lesion(7). There may also be genetic factors that influence the rate of transformation to neoplasia in pterygia among different populations(15). In our group of patients, there was no suspicion of OSSN before surgery. Evidence of clinical OSSN can be seen in the slit-lamp if the patient presents well-demarcated corneal epithelial clouding emanating from the head of the pterygium(9).
The presence of unexpected pterygium-associated OSSN poses a management dilemma because routine surgeries for pterygium and OSSN differ. In addition, information regarding the best approach for its management is limited. All cases (n=20) of unexpected OSSN with pterygia previously described by Oellers et al. were treated with simple excision. Some of these patients received postoperative interferon eye drops or underwent a second excision using wider margins following the OSSN diagnosis. These authors have suggested that surgeons in geographical regions with high UV radiation should have a higher index of suspicion for OSSN when evaluating and treating pterygia(14).
Interestingly, according to Galor et al., a foci of OSSN within a clinically diagnosed pterygium was associated with a decreased risk of OSSN recurrence, when compared to that of isolated OSSN(13). However, other authors have found that the recurrence rates for unexpected OSSN with pterygia are comparable to those for isolated OSSN(14). Further studies with a larger number of patients are needed to evaluate whether adjuvant measures for unsuspected and subclinical OSSN with pterygia can decrease the recurrence rate.
Two studies have described the finding of pigmented ocular lesions, including premalignant primary acquired melanosis (PAM) with atypia within pterygia(8,10). However, no atypical melanocytic lesions were found by histopathology in our patients and this condition appears to be rare.
As stratospheric ozone depletion can potentially result in further increases in the incidence of pterygia and OSSN(3), it is advisable to conduct a histopathological evaluation of all excised conjunctival lesions(9). Premalignant OSSN or PAM with atypia may coexist with pterygia and these conditions could remain undiagnosed if the excised pterygium is discarded(10). Furthermore, we suggest that patients in whom atypical cells are noted should be examined at more frequent intervals in order to identify possible carcinomatous changes at an early stage(9).
IC analysis demonstrated close agreement with our histopathological study in the identification of atypical epithelial cells in the superficial layers of the epithelium. Using histopathology, 40.6% of the pterygia specimens received a diagnosis of unsuspected and subclinical foci of OSSN. This value is very close to the 39.5% found by Leite(12)in his sample of Brazilian pterygia specimens, evaluated after surgery in the city of Campo Grande. According to his study there was a correlation between pterygium-associated OSSN and patients who reported more than 10 years of solar exposure and who were exposed to sunlight for more than eight hours per day. Nevertheless, despite this subjective patient information, an objective measure involving solar elastosis was not found to be correlated to OSSN(12). In our study, the duration of solar exposure was not available for analysis and, similar to our observation, inflammation on histopathology was not correlated to OSSN in the pterygia specimens(12).
One limitation of this study was the small number of patients. Since our purpose was to demonstrate that IC can accurately detect the foci of unsuspected OSSN in pterygia, further investigations using a larger number of patients are warranted. The prevalence discrepancy observed between the studies shown in table 1 and the Brazilian pterygia specimens may be the result of different geographic locations, different levels of UV radiation exposure, and genetic factors that influence the rate of transformation to OSSN in pterygia among different populations and races(15). This study´s strength was its prospective nature and that it represents the first report of subclinical OSSN characterized by atypical epithelial cells detected by IC in pterygia before surgical treatment.
In conclusion, although OSSN and pterygium share common risk factors, a definitive link of progression remains unproven. The value of IC lies in its capacity to detect both the presence and extent of OSSN when the clinical diagnosis is difficult, and to detect subclinical disease and to follow-up previously diagnosed disease(16,21).




 English PDF
English PDF
 Print
Print
 Send this article by email
Send this article by email
 How to cite this article
How to cite this article
 Submit a comment
Submit a comment
 Mendeley
Mendeley
 Scielo
Scielo
 Pocket
Pocket
 Share on Linkedin
Share on Linkedin

